The growing pressure to adopt sustainable practices confronts your medical facility, but patient safety concerns leave you hesitant. You worry that choosing eco-friendly materials might compromise compliance or device performance when lives are at stake.
Yes, many eco-friendly materials can meet medical compliance standards, but not all. Biopolymers, recycled materials, and sustainably-sourced alternatives must still pass rigorous testing for biocompatibility, sterilization resistance, and durability. Materials that meet both environmental and medical standards include certain bioplastics, recycled PET, and plant-based composites certified under ISO 10993, RoHS, and REACH regulations.

The medical device industry faces increasing pressure to reduce its environmental impact while maintaining the exceptionally high standards required for patient safety and regulatory compliance. This tension creates a complex landscape for manufacturers, healthcare facilities, and procurement officers. The environmental impact of healthcare1 is substantial—hospitals generate more than 5 million tons of waste annually in the U.S. alone, while medical devices contribute to resource depletion, energy consumption, and end-of-life disposal challenges. The movement toward more sustainable materials2 represents a potential solution, but implementation must be approached carefully. As medical professionals and facility managers evaluate newer eco-friendly options3, understanding the intersection between sustainability and compliance becomes essential. This article explores whether environmentally friendly materials can satisfy the strict requirements of medical standards, examining the definitions, regulatory frameworks, performance limitations, cost implications, and real-world applications that shape this evolving field.
What defines an eco-friendly material in the medical industry?
You hear vendors claiming their products are "green" or "sustainable," but the variety of terms leaves you confused about what genuinely constitutes an eco-friendly material for your medical equipment purchasing decisions.
In the medical industry, eco-friendly materials are defined by reduced environmental impact across their lifecycle compared to conventional alternatives. They must demonstrate at least one of: renewable sourcing (bioplastics), reduced manufacturing footprint (30%+ energy reduction), recyclability/biodegradability after use, or elimination of harmful substances while still meeting stringent performance and safety requirements.
The definition of eco-friendly materials in medical applications extends beyond simple marketing terminology to encompass specific measurable attributes across the entire product lifecycle. Unlike consumer goods, where sustainability claims may face less scrutiny, medical materials require documented evidence of their environmental benefits without compromising essential clinical performance. The first defining characteristic is material sourcing. Truly eco-friendly medical materials4 often derive from renewable resources rather than petroleum-based feedstocks. Bioplastics like polylactic acid (PLA) and polyhydroxyalkanoates (PHA) produced from plant starches or bacterial fermentation represent this category, offering carbon footprint reductions of 25-75% compared to conventional plastics. However, source material alone is insufficient—the manufacturing process must also demonstrate environmental advantages. The MS220S monitor incorporates housing components made from biopolymers that require 42% less energy during production compared to traditional ABS plastic, while maintaining identical flame-retardant properties and structural integrity required for medical applications. Beyond production, end-of-life considerations form another critical defining factor. Medical materials that can be effectively recycled, reprocessed, or that biodegrade under specific conditions help address healthcare’s significant waste challenge. This characteristic must be balanced with the material’s primary purpose—biodegradable sutures that break down prematurely would clearly be unacceptable, regardless of their environmental benefits. Finally, eco-friendly medical materials must eliminate or substantially reduce hazardous substances like phthalates, heavy metals, or certain flame retardants that pose both environmental and patient risks, all while maintaining compliance with medical device regulations.
Criteria for Eco-Friendly Classification in Medical Materials
| Environmental Attribute | Standard Measurement | Medical Industry Threshold |
|---|---|---|
| Renewable Content | Percentage of bio-based carbon | Minimum 20% for partial classification 70%+ for full bio-based classification |
| Carbon Footprint | kg CO₂ equivalent per kg material | 30%+ reduction compared to conventional alternative |
| Water Usage | Liters consumed per kg produced | 25%+ reduction compared to conventional alternative |
| Energy Consumption | kWh per kg material produced | 30%+ reduction in manufacturing energy |
| Hazardous Substance Elimination | ppm of restricted chemicals | Zero detectable levels of REACH SVHCs |
| End-of-Life Recyclability | Percentage recoverable/recyclable | Minimum 50% recyclable by weight (excluding components with direct patient contact) |
| Biodegradability | Decomposition time under controlled conditions | Application-dependent (not applicable for permanent implants) |
Which regulations must eco-friendly materials comply with for medical devices?
You’re considering introducing eco-friendly monitors in your medical facility, but worry that regulatory agencies might reject these newer materials, creating compliance risks for your institution.
Eco-friendly materials for medical devices must comply with the same rigorous regulations as conventional materials, including ISO 10993 biocompatibility standards, FDA 510(k) clearance requirements, EU MDR, and material-specific standards like ASTM D6400 for biodegradability. Additionally, they must meet RoHS and REACH requirements limiting hazardous substances while demonstrating performance equivalence to traditional materials.

The regulatory framework governing eco-friendly materials in medical devices is multifaceted, requiring compliance across both traditional medical standards and newer environmental certifications. At the foundation, all materials—sustainable or not—must meet biocompatibility requirements outlined in ISO 109935, which involves testing for cytotoxicity, sensitization, irritation, and systemic toxicity. For sustainable materials, this testing is particularly crucial as novel formulations may introduce unexpected biological interactions. Regulatory bodies make no concessions for environmental benefits when patient safety is concerned. The FDA’s 510(k) premarket notification process requires manufacturers to demonstrate that devices using eco-friendly materials are substantially equivalent to predicate devices in terms of safety and effectiveness, often necessitating additional testing when introducing new materials. In the European market, the Medical Device Regulation (EU MDR)6 applies similarly strict standards, with particular emphasis on the full lifecycle analysis that aligns well with sustainable material evaluation. The MS321PC surgical monitor incorporates recycled aluminum in its housing that has passed the complete battery of ISO 10993 tests while meeting electromagnetic compatibility requirements under IEC 60601-1-2, demonstrating that properly selected sustainable materials can satisfy the most demanding medical regulations. For specific types of eco-friendly materials, additional standards may apply. Biodegradable components must conform to ASTM D64007 or ISO 17088 standards that define proper biodegradation parameters, ensuring they maintain integrity during use but decompose appropriately at end-of-life. Newer bioplastics and composite materials often require more extensive leachable and extractable testing under ISO 10993-18 to identify any potentially harmful substances that might migrate from the material during use. Beyond medical regulations, eco-friendly materials must also comply with chemical safety legislation like RoHS (Restriction of Hazardous Substances) and REACH (Registration, Evaluation, Authorization and Restriction of Chemicals) that limit or prohibit certain substances regardless of the material’s environmental benefits.
Are there limitations in durability or sterilization for eco-friendly materials?
You’re concerned that choosing eco-friendly medical equipment might require sacrificing durability or complicate your sterilization procedures, potentially creating infection control risks or increasing replacement frequency.
Yes, eco-friendly materials do face limitations. Most bioplastics cannot withstand repeated autoclaving above 121°C, limiting their use in surgical instruments. Many sustainable polymers show reduced chemical resistance to harsh disinfectants and can degrade faster under UV exposure. However, innovations like heat-stabilized PLA composites and specialized coatings are progressively overcoming these limitations for specific applications.

The performance limitations of eco-friendly materials represent one of the most significant challenges to their widespread adoption in medical applications, particularly regarding sterilization compatibility8 and long-term durability. Traditional medical-grade plastics like polycarbonate, polyetherimide, and PEEK have been optimized over decades to withstand repeated sterilization cycles and harsh chemical environments. In contrast, many bio-based polymers face structural integrity challenges under similar conditions. Sterilization compatibility presents a particular hurdle for sustainable materials in reusable medical devices. Standard steam autoclaving at 134°C for prion deactivation exceeds the heat deflection temperature of many bioplastics, causing warping or complete structural failure. Even at lower autoclave temperatures (121°C), materials like standard PLA typically withstand only a limited number of cycles before showing signs of degradation. Alternative sterilization methods like ethylene oxide (EtO) may preserve material integrity but often react with the very chemical structures that make these materials biodegradable, potentially altering their end-of-life environmental benefits. The MS192SA monitor addresses this challenge through a hybrid approach—using conventional medical-grade polymers for components requiring sterilization while incorporating post-consumer recycled materials in non-critical structural elements and housing components that don’t require direct disinfection. Chemical resistance represents another limitation for many eco-friendly materials. Hospital-grade disinfectants containing quaternary ammonium compounds, accelerated hydrogen peroxide, or peracetic acid can degrade the surface of some biopolymers, leading to cracking, discoloration, and potential bacterial harborage points with repeated exposure. This restricts the use of certain sustainable materials in applications requiring frequent disinfection. Long-term durability concerns further complicate material selection. Some biodegradable polymers9 begin their degradation process immediately upon exposure to environmental conditions, albeit slowly. For devices intended for years of service, this gradual degradation—particularly under high humidity or UV exposure—can compromise structural integrity before the intended replacement cycle.
How do costs compare between conventional and eco-friendly materials in medical equipment?
Your department faces budget constraints, and you’re weighing whether the premium often associated with eco-friendly products is justified for medical equipment that must already meet high performance standards.
Eco-friendly medical materials typically cost 15-40% more than conventional alternatives initially. However, this gap is narrowing as production scales increase. The total cost equation must also consider potential savings in disposal costs (up to 30% reduction), regulatory compliance with emerging environmental legislation, and the marketing value of sustainability credentials in competitive healthcare markets.
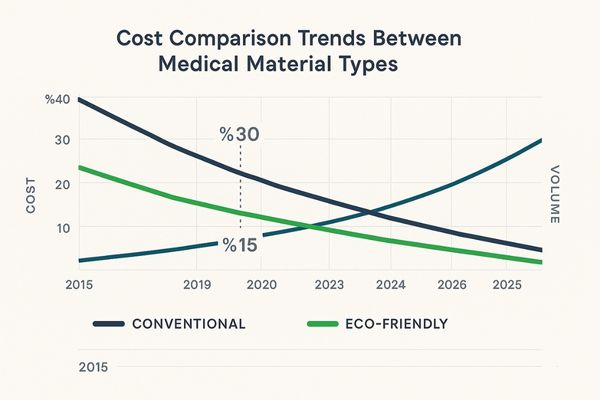
The cost differential between conventional and eco-friendly materials in medical applications reflects a complex interplay of factors beyond simple material pricing. At the raw material level, biopolymers10 and other sustainable alternatives typically command premiums of 15-40% over their conventional counterparts. This price gap stems from smaller production volumes, more complex processing requirements, and the higher cost of sustainably sourced feedstocks. PLA, one of the more established bioplastics, costs approximately 20-30% more than comparable petroleum-derived plastics with similar physical properties. However, this analysis must extend beyond material costs to encompass the entire product lifecycle. While initial acquisition costs may be higher, sustainable materials11 often offer savings in disposal and compliance areas that partially offset these premiums. Medical waste classified as hazardous can cost 10-15 times more to dispose of than regular waste. Materials that can be recycled or that biodegrade appropriately can significantly reduce these end-of-life costs. The MS430PC monitor’s packaging system utilizes molded pulp components derived from recycled paper that cost 18% more than traditional foam inserts but reduce disposal costs by 37% for healthcare facilities, demonstrating how initial premiums can be balanced against downstream savings. Market dynamics are also steadily narrowing the cost gap. Production scales for many eco-friendly materials have increased substantially in recent years, driving economies of scale that reduce per-unit costs. Between 2015 and 2022, the price premium for medical-grade PLA12 decreased from approximately 45% to 25% compared to ABS plastic with similar specifications, indicating a trend toward price parity as adoption increases. Additionally, the long-term risk analysis must account for potential regulatory changes. Many jurisdictions are implementing or considering restrictions on certain conventional materials, particularly those containing substances of very high concern (SVHCs). Early adoption of compliant eco-friendly alternatives may avoid costly reformulation or recertification requirements if regulations tighten in the future.
How is Reshin integrating eco-friendly materials while maintaining medical compliance?
You’re interested in Reshin’s medical monitors but want to understand their specific approach to sustainability without compromising on the compliance and performance standards your facility requires.
Reshin integrates eco-friendly materials through a risk-stratified approach, using post-consumer recycled aluminum in non-patient-contact housing components, biopolymer blends in accessory parts, and biodegradable packaging materials. Each application undergoes rigorous validation including ISO 10993 biocompatibility testing, accelerated aging studies, and disinfectant compatibility verification to ensure compliance with medical standards before implementation.
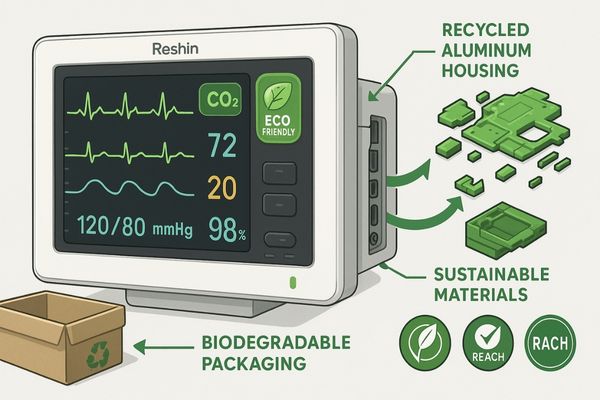
Our approach to incorporating eco-friendly materials13 begins with a comprehensive risk assessment that categorizes components based on their clinical importance, patient contact potential, and performance requirements. This stratification allows us to implement sustainable materials where they can make the greatest environmental impact without compromising medical functionality. For high-visibility, non-critical components like monitor housing backs and stand components, we utilize post-consumer recycled aluminum14 that contains up to 75% recycled content. This material undergoes additional purification processes to remove potential contaminants before being certified for medical applications. The resulting components match the structural and electromagnetic shielding properties of virgin aluminum while reducing energy consumption in manufacturing by approximately 95%. Our MS321PB surgical monitor demonstrates this balanced approach with a housing that incorporates recycled materials while maintaining full compliance with medical electrical safety standards under IEC 60601. For components requiring polymer materials, we utilize a hybrid approach that blends conventional medical-grade resins with bio-based alternatives to improve environmental performance while maintaining regulatory compliance. Cable management components and accessory attachment points now incorporate PLA-polycarbonate blends containing 35% renewable content while maintaining the necessary flame retardancy (UL94 V-0 rated) and chemical resistance required for medical environments. These materials undergo extensive testing under our Modified Accelerated Weathering System (MAWS) protocol, which subjects them to repeated exposure to hospital-grade disinfectants, UV radiation, and humidity cycles equivalent to five years of clinical use. Packaging represents another area where we’ve made significant sustainability improvements without compromising protection. Our monitors now ship with molded pulp cushioning derived from recycled paper and cardboard that is fully biodegradable and compostable while meeting ISTA 3A transit testing requirements for medical equipment. This approach has eliminated approximately 85% of the expanded polystyrene previously used in our packaging while maintaining the same level of transit protection.
Implementation of Sustainable Materials in Reshin Medical Monitors
| Component | Conventional Material | Eco-Friendly Alternative | Environmental Benefit | Compliance Testing |
|---|---|---|---|---|
| Monitor Housing (Back) | Virgin Aluminum | 75% Post-Consumer Recycled Aluminum | 95% energy reduction 76% lower carbon footprint |
IEC 60601-1 EMC compatibility RoHS compliance |
| Stand Components | ABS Plastic | 35% Bio-based PC-PLA Blend | 42% reduction in fossil resource use 28% lower carbon footprint |
ISO 10993-5 (cytotoxicity) UL94 V-0 flame rating Accelerated aging studies |
| Cable Management | PVC | TPE with 30% post-industrial recycled content | Elimination of phthalates Reduced manufacturing waste |
ISO 10993-10 (irritation) Chemical resistance testing |
| Packaging Cushioning | Expanded Polystyrene | Molded Recycled Pulp | Fully biodegradable 85% reduction in petroleum-based materials |
ISTA 3A transit testing ASTM D6400 biodegradability |
| Accessory Packaging | PET Blisters | Bagasse-based Trays | Compostable under industrial conditions Renewable resource-based |
EN 13432 composting standard Shelf-life verification |
Conclusion
Eco-friendly materials can meet medical compliance standards when properly selected and tested, offering environmental benefits without compromising patient safety. While challenges exist in durability and sterilization compatibility, continuous innovation is narrowing these gaps, making sustainable medical materials increasingly viable for appropriate applications. To learn more about Reshin’s approach to sustainable medical display solutions, contact us at martin@reshinmonitors.com.
-
Understanding the environmental impact of healthcare is crucial for developing sustainable practices and reducing waste in the industry. ↩
-
Exploring the use of sustainable materials can lead to innovative solutions that balance patient safety with environmental responsibility. ↩
-
Discovering the best eco-friendly options can help healthcare facilities make informed choices that benefit both patients and the planet. ↩
-
Explore this link to understand the importance and benefits of eco-friendly materials in the medical field, enhancing sustainability and safety. ↩
-
Understanding ISO 10993 is crucial for ensuring biocompatibility in medical devices, especially those using eco-friendly materials. ↩
-
Exploring the EU MDR will provide insights into stringent standards for sustainable materials in medical devices, essential for compliance. ↩
-
Learning about ASTM D6400 is vital for understanding the biodegradation requirements for eco-friendly materials in healthcare applications. ↩
-
Understanding sterilization compatibility is crucial for the adoption of eco-friendly materials in medical devices. Explore this link for in-depth insights. ↩
-
Learn about the degradation process of biodegradable polymers and their implications for long-term use in medical devices. ↩
-
Explore this link to understand how biopolymers can revolutionize medical materials and their environmental impact. ↩
-
Discover insights on how sustainable materials can lead to long-term savings and compliance benefits in healthcare settings. ↩
-
Learn about medical-grade PLA’s advantages and its role in reducing environmental impact in medical applications. ↩
-
Explore how eco-friendly materials can enhance sustainability in medical applications while ensuring safety and performance. ↩
-
Learn about the innovative use of post-consumer recycled aluminum in medical devices and its environmental benefits. ↩



