Medical-Grade Monitor Manufacturer—Surgical & Diagnostic Displays
Trusted Medical-Grade Monitor Manufacturer for Medical Integrators and Partners
Partnerships with 300+ companies across surgical displays, diagnostic monitors, and cath lab monitors have been sustained long-term, which reduces vendor risk and keeps imaging quality consistent. Trusted by global medical system manufacturers















Trusted by global medical system manufacturers for DICOM-compliant diagnostic displays and surgical imaging monitors, including WDM,Olympus, Pentax, and Karl Storz. See referenced brands & standards →
Inside Reshin Factory – Innovating Medical-Grade Monitor Manufacturing
- Chinese national high-tech medical-grade monitor manufacturer.
- The largest medical grade monitors manufacturer in China.
- 200+ engineers with 20 years of display expertise.
- Trusted by 100,000+ hospitals using diagnostic and surgical monitors.
- Rich OEM/ODM projects with global medical brands.
Our factory is built for consistent output, controlled revisions, and traceability options to support long-term medical grade monitors programs.With in-house R&D, mass production capability, and strict quality control, Reshin delivers reliable OEM and ODM medical displays for global partners, ensuring long-term consistency and clinical compliance.




Manufacturer Capabilities for Medical Grade Monitors Programs
Advanced R&D and manufacturing are integrated end-to-end—from imaging algorithms and optical bonding to assembly and aging tests—supporting PACS diagnostic monitors and surgical imaging systems with stable quality and consistency.—so each surgical display, radiology monitor, and medical all-in-one display meets global clinical standards with fewer integration surprises and lower validation costs.

Repeatable builds and stable output for medical-grade monitor programs, supporting long-term supply of PACS diagnostic monitors and surgical imaging displays.
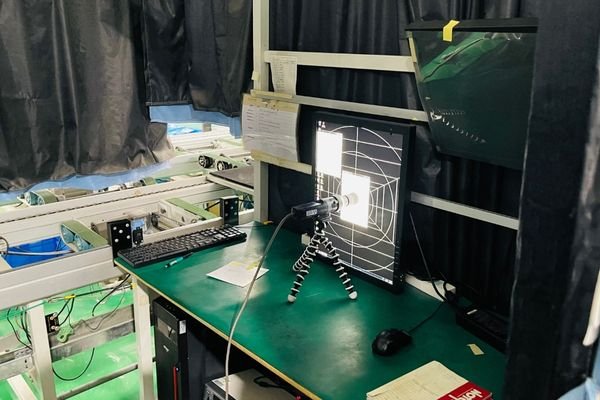
Support verification and calibration records to ensure consistent image performance across PACS diagnostic monitors and surgical imaging systems in clinical use.
Calibration Consistency

Offer optical bonding and front-glass options evaluated for durability, cleaning, and improved visibility in operating rooms and surgical imaging environments.
Optical Bonding Options
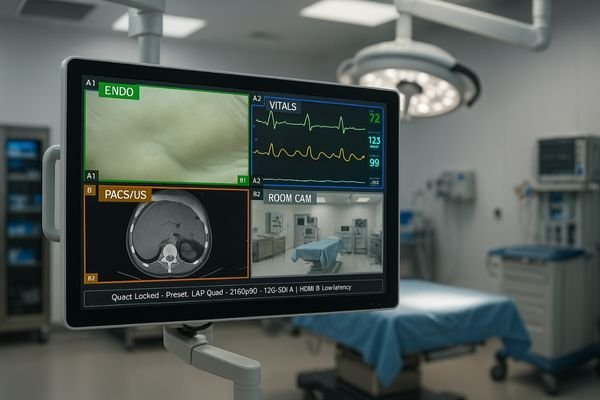
Tune signal integration for endoscopy systems and imaging devices, ensuring stable input performance for surgical and PACS monitors.
Signal Integration Support

Quality control and reliability verification processes designed to reduce field failure risks in regulated diagnostic and surgical imaging applications.
Quality Control and Reliability Verification

Lifecycle program support covering revision control, documentation, and long-term supply for OEM PACS diagnostic and surgical imaging systems.
Lifecycle Program Support
Start a Manufacturer Evaluation
Send requirements and target markets; we confirm feasibility, verification needs, and a production approach for medical grade monitors.
Manufacturer Program Process for Medical Grade Monitors

Step 1:Requirements Alignment
Confirm interfaces, use environment, target markets, and acceptance criteria for your program.
Step 2:Feasibility Evaluation
Review customization scope, risks, and manufacturability before locking a practical technical route.


Step 3: Sample Build
Provide samples for integration testing, image checks, and functional validation in your system.
Step 4:Verification and Freeze
Freeze key specs and verify stability to avoid uncontrolled changes during production ramp.


Step 5: Pilot Production
Run small batches to validate process stability, QC checkpoints, and delivery readiness.
Step 6:Volume Supply
Support long-term supply with controlled revisions, documentation cooperation, and stable delivery planning.

Manufacturing Program Overview
Manufacturer Support for Medical Grade Monitors Programs

- Specification Control
Lock key specifications and acceptance criteria to keep production consistent across shipments.
- Integration Coordination
Support engineers with interface behavior, signal stability, and system-level integration questions.
- Documentation Support
Prepare standard documents and cooperate on compliance needs for local registration workflows.
- QC and Traceability
Provide QC checkpoints and traceability options based on program risk and market requirements.
- Issue Prevention
Use structured feedback loops to reduce field issues and shorten troubleshooting cycles.
- Lifecycle Cooperation
Manage controlled revisions to support long-term supply without disrupting customer deployments.
Quality System for Medical Grade Monitors Manufacturing
Production and quality processes for medical grade monitors are controlled under ISO 13485, simplifying vendor audits and regulatory review.
CE MDR and IEC60601 compliance help secure safe, reliable performance and support faster market access.
PACS medical monitors are aligned with DIN 6868-175 to maintain diagnostic accuracy and cross-site consistency.
Conclusion: Selecting suppliers with proven certifications and hospital references reduces procurement risk and supports long-term ROI.
Get a Manufacturer Response
Share program type, size, interfaces, and target markets for medical grade monitors. We will respond with a manufacturing-ready proposal and next steps.
Yes. Reshin is a medical grade monitors Manufacturer supporting regulated surgical and diagnostic display programs.
We support documentation alignment for medical grade monitors; registration responsibility remains with the local legal entity.
We manufacture medical grade monitors for surgical imaging, endoscopy workflows, and diagnostic imaging applications.
We apply defined QC checkpoints and final functional verification to keep medical grade monitors output consistent.
Process controls and verification steps are used to reduce variation across medical grade monitors production lots.
Yes. Verification records can be prepared for medical grade monitors based on program needs and market requirements.
We verify input behavior and mode stability for medical grade monitors used with endoscopy and routing conditions.
Yes. Adjustments are supported within a controlled framework to keep medical grade monitors repeatable for long supply.
