A brand-new surgical display looks perfect until its first cleaning. After a standard wipe-down with disinfectant, the screen hazes over, compromising the surgeon’s view during a critical procedure.
True durability in the operating room is defined by ingress protection (IP) ratings, verified chemical resistance against disinfectants, and robust sealing. These details ensure long-term hygiene, image clarity, and device reliability.

When selecting equipment for the operating room, it is easy to focus on primary performance specifications like resolution, brightness, and color accuracy. While these are undeniably important, they only tell half the story. The OR is an unforgiving environment, subject to fluids, airborne particles, and a relentless regimen of cleaning with harsh chemical disinfectants. In this setting, the small, often-overlooked details of a display’s physical construction—its waterproofing, dustproofing, and chemical resistance1—are what ultimately determine its long-term viability. A display that cannot withstand the daily realities of the OR environment will inevitably fail, leading to compromised patient safety, operational disruptions, and a high total cost of ownership. Reliability is engineered from the outside in, starting with a design that is fundamentally resilient to its surroundings.
What waterproofing, dustproofing, and disinfectant resistance mean
Water and dust protection are defined by specific Ingress Protection (IP) ratings, while disinfectant resistance is verified by testing a device’s surfaces against repeated wipe cycles with specific chemical agents.
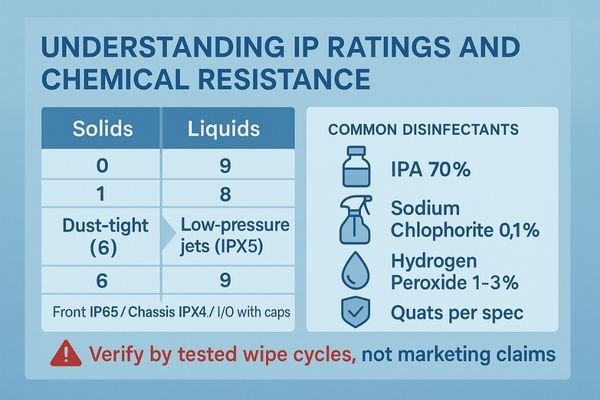
Start with verifiable definitions and standards, not marketing claims or single-surface ratings. Water and dust protection are defined by Ingress Protection (IP) ratings2 (IEC 60529). The first digit (0–6) covers solids; the second (0–9) covers liquids. Example benchmarks to verify with the vendor: Front IP65 for wash-down, plus explicit chassis and I/O protection ratings (e.g., Front IP65 / Chassis IPx4 / I/O with caps IPx5). Disinfectant resistance is separate from IP and must be validated by repeated wipe cycles using named agents and concentrations.
Understanding IP Ratings
IP ratings quantify dust and water ingress tolerance. A “6” for solids indicates dust-tight; a “5” for liquids (IPX5) indicates resistance to low-pressure jets. Confirm whether the rating applies only to the front bezel or to the whole unit, including rear housing and connectors.
Defining Chemical Resistance
Chemical resistance refers to the durability of coatings, gaskets, adhesives, and cover glass against approved disinfectants. Request an approved/forbidden list and tested wipe cycles, for example: IPA 70% ≥1,000 cycles; NaOCl 0.1% ≥500 cycles; H₂O₂ 1–3% ≥500 cycles; quats per vendor concentration ≥1,000 cycles. Surfaces should remain free of haze, cracking, yellowing, or peeling.
Why these small details matter in the OR
Fluids and residues can creep into unsealed gaps, harsh disinfectants erode surfaces, and accumulated dust can obscure the image. These details directly impact hygiene, image quality, and device lifespan.

Design for fluids, residues, and frequent disinfection3; otherwise, image quality, hygiene, and lifespan will degrade. Fluids and rinse agents can pool and wick into seams; frequent wipes with harsh chemistries abrade and erode surfaces; dust and films add glare and reduce contrast. Over time, these stressors determine whether a device remains a reliable tool or becomes a liability.
The Cumulative Effect of Cleaning
OR suites are disinfected multiple times daily. Without resistant materials and sealed construction, routine cycles produce surface haze and reduced contrast. Dust and aerosols can infiltrate non-sealed enclosures, while external films increase reflections, forcing clinicians to second-guess visual cues.
Risks when protection is inadequate
Liquid ingress can cause electrical shorts and blackouts, post-disinfectant haze degrades image contrast, and aging seals can lead to I/O instability, all contributing to higher infection risk and unplanned downtime.

Expect clinical, technical, and operational consequences that often surface at the worst possible moment.
Clinical and Patient Safety Risks4
Liquid ingress can black out a display mid-procedure. Haze and contrast loss obscure tissue planes and subtle cues. Material degradation creates crevices that harbor bioburden, undermining sterilization and elevating HAI risk.
Technical Failure and Downtime
Gaskets and adhesives that are not chemically resistant become brittle and crack under thermal cycles, allowing dust and moisture into the chassis. Intermittent I/O and eventual board failures lead to unplanned downtime, cancellations, and emergency service calls.
The solution: sealing, optical bonding, chemical resistance
A comprehensive solution combines unit-level IP-rated design, AR-hardened optically bonded cover glass, and materials with verified chemical resistance, all supported by a clear Standard Operating Procedure for cleaning.
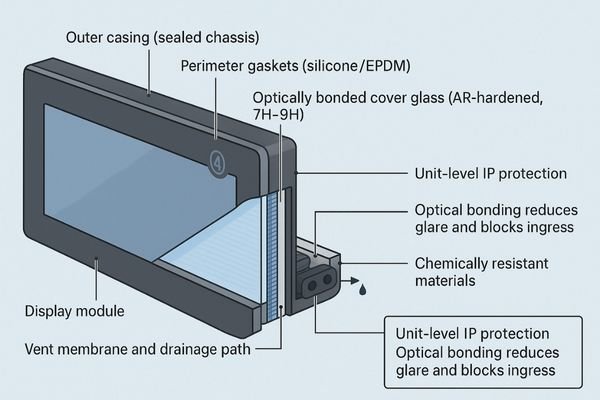
Engineer a whole-unit protection strategy with clear acceptance criteria and traceable records.
Sealing (design goal, practices, acceptance)
Design goal: unit-level ingress protection.
Key practices: perimeter gaskets (e.g., silicone/EPDM), vent membranes, drainage paths, capped I/O, membrane keys or internal OSD.
Acceptance: dust and spray/wash tests with visual logs; declare Front/Chassis/I-O IP ratings.
Optical bonding (design goal, practices, acceptance)
Design goal: block liquid ingress and boost contrast while reducing glare and internal condensation.
Key practices: full optical bonding; AR-hardened cover glass (e.g., 7H–9H); adhesive thickness/clarity (e.g., 0.5–1.0 mm, >92% transmission).
Acceptance: post-bond haze/contrast targets and edge-seep checks documented.
Verified chemical resistance5 (design goal, practices, acceptance)
Design goal: no haze, cracking, or peeling after repeated disinfection.
Key practices: approved/forbidden agent list with concentrations and wipe cycles (e.g., IPA 70% ≥1,000; NaOCl 0.1% ≥500; H₂O₂ 1–3% ≥500; quats per spec ≥1,000).
Acceptance: before/after appearance, contrast delta, and touch reliability (if applicable) with signed test logs.
Standardized cleaning SOP (design goal, practices, acceptance)
Design goal: consistent, traceable care with minimal damage risk.
Key practices: wipe order, dwell time (e.g., 1–3 min), wipe pressure/velocity (e.g., ≤1 kgf, S-pattern), drying time (e.g., ≤2 min), routine inspection cadence (e.g., weekly/quarterly).
Acceptance: timestamped records with operator signature and findings.
Disinfectant & Cleaning Acceptance Log (template)
| Date | Agent & concentration | Cycles | Dwell (min) | Dry (min) | Visual score | Contrast Δ | Notes | Sign |
|---|
Recommended Resilient Surgical Displays
| Model | Size / Resolution | Key Protective Features |
|---|---|---|
| MS192SA | 19" HD | Compact and easy to disinfect, ideal for crowded operating tables and mobile carts. |
| MS322PB | 32" 4K | All-around 4K display with a focus on durability and cleanability for main surgical viewing. |
| MS270P | 27" FHD | Simple to maintain, suitable for auxiliary viewing, teaching, and information display. |
| MS550P | 55" 4K | Large screen with an easy-to-clean front surface for clear team viewing from a distance. |
For specs or disinfectant whitelist, contact info@reshinmonitors.com.
Business value: safer, steadier, lower TCO
Displays that maintain stable image quality under frequent cleaning reduce clinical risk, while fewer failures from ingress or corrosion mean less downtime and a lower total cost of ownership (TCO).
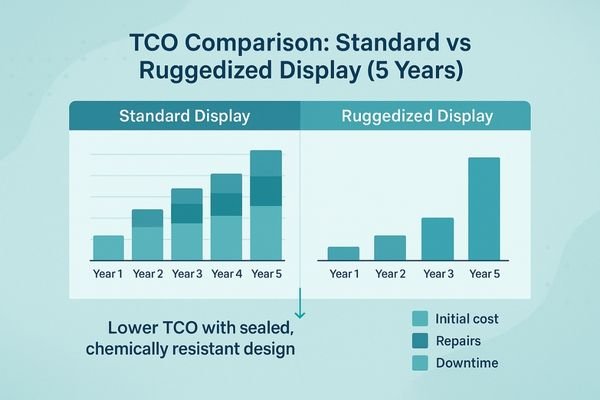
Tie engineering choices to measurable outcomes: fewer failures, fewer cancellations, smoother audits. Well-protected displays preserve contrast and clarity after hundreds of cleaning cycles, reducing clinical risk and rework. Lower ingress and corrosion failures mean less downtime and fewer emergency service calls. Traceable chemical-resistance data and a clear cleaning SOP simplify procurement, acceptance, and audits, lowering total cost of ownership over the device’s life.
FAQ
Is an IP65 rating sufficient for a surgical display?
It is often a good benchmark for front-panel wash-down protection, but it may not be enough. You must verify the protection level for the entire unit, including the rear housing and I/O connections. Furthermore, IP rating does not cover disinfectant resistance, which must be validated separately.
Does optical bonding increase the risk of the screen shattering?
No, it typically does the opposite. Using a hardened Anti-Reflective (AR) cover glass provides a strong outer layer. The elastic adhesive used in the bonding process helps to disperse the force of an impact across a wider area, reducing the risk of a fracture. It also prevents glass shards from scattering if a catastrophic break does occur.
Which cleaning agents are the harshest on displays?
Generally, high-concentration chlorine-based solutions, peroxide, and strong alkaline or acidic agents are the most damaging. It is critical to follow the display manufacturer’s whitelist of approved chemicals and recommended concentrations and wipe cycles. Promptly drying the surface after the required dwell time is also essential to prevent damage.
Conclusion
Combine unit-level IP design, AR-bonded glass, and verified chemical resistance with documented cleaning SOPs to make durability measurable, repeatable, and auditable for long-term OR hygiene and image quality.
-
Understanding these practices can help ensure the longevity and reliability of medical displays in challenging environments. ↩
-
Understanding IP ratings is crucial for ensuring the durability and reliability of devices in various environments. ↩
-
Explore this link to learn effective disinfection strategies that ensure hygiene and maintain equipment performance. ↩
-
Understanding these risks is crucial for improving patient safety and device reliability. ↩
-
Learn about the rigorous testing methods for Verified chemical resistance to ensure device durability against disinfectants. ↩



