Medical displays are a big investment. It’s tempting to look at cheaper consumer screens and wonder. Can a few tweaks make them hospital-ready?
Modifying consumer-grade displays to meet stringent medical standards is generally not feasible or cost-effective. The fundamental differences in performance, design, regulatory requirements, and reliability make such conversions impractical and potentially unsafe for diagnostic use in healthcare environments.
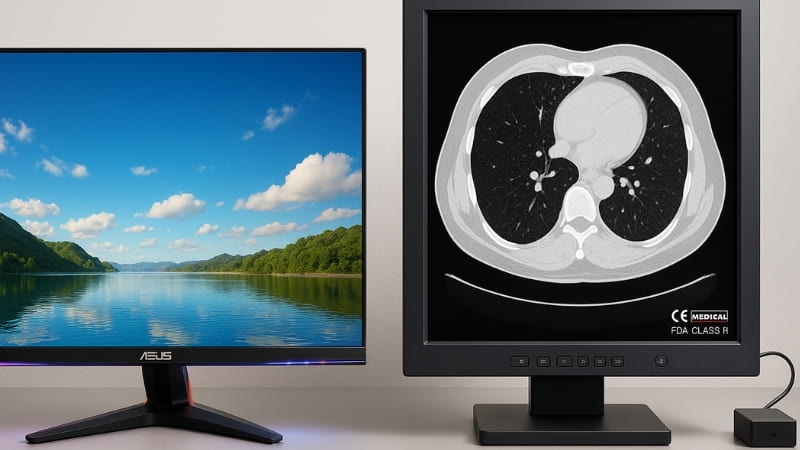
I’ve been in the display business for a long time, especially here at Reshin focusing on medical technology. This question comes up more often than you might think. Many people see the price difference and assume a few upgrades could bridge the gap. However, the differences are far more than skin deep.
It’s important to understand that medical displays are purpose-built tools. They are designed from the ground up for a very specific and critical task: accurate medical diagnosis. Let’s explore why trying to adapt a consumer product for this role is usually a dead end.
What technical parameters of consumer-grade displays differ most significantly from medical standards?
You might think a screen is a screen. But when lives are on the line, details matter. What makes a medical screen so different inside?
Consumer displays typically prioritize vibrant colors and fast refresh rates for entertainment. Medical displays, however, demand exceptional grayscale accuracy, high contrast ratios for subtle detail, consistent brightness, long-term stability, and compliance with standards like DICOM Part 14 for image consistency.
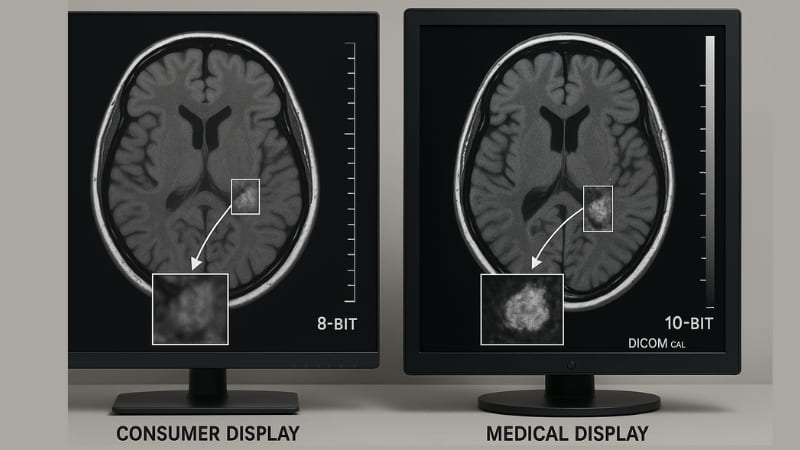
From my experience at Reshin, the core performance differences are vast. Consumer-grade displays are designed for a mass market. They aim for pleasing aesthetics – bright, saturated colors for movies and games, and often glossy screens that look good in a showroom. However, these characteristics are often counterproductive in a medical setting. For example, consumer displays are prone to color distortion. They might make an image look more vivid, but this isn’t what a radiologist needs. They need to see the true, subtle variations in tissue density, which are represented by minute changes in grayscale.
Medical displays, particularly those we develop at Reshin, have extremely high requirements for grayscale reproduction, often supporting 10-bit or 12-bit grayscale for thousands of distinct shades. They also need high, stable contrast ratios and specific luminance levels to ensure that faint lesions or anomalies are visible. Consumer panels usually offer 8-bit color/grayscale and their brightness can fluctuate. It’s very difficult, if not impossible, to modify the fundamental panel technology and backlight systems of a consumer display to consistently achieve the precise image representation required for professional medical diagnosis. The internal processing and calibration capabilities are just not built for it.
Key Parameter Differences
| Parameter | Consumer-Grade Display | Medical-Grade Display (e.g., Radiology) | Why it Matters for Medical |
|---|---|---|---|
| Grayscale Depth | Typically 8-bit (256 shades) | 10-bit, 12-bit+ (1024-4096+ shades) | Crucial for subtle tissue differentiation |
| Luminance | Variable, often lower sustained brightness | Higher, stable, calibrated (e.g., 350+ cd/m²) | Ensures visibility of faint details |
| Contrast Ratio | Optimized for visual appeal, can be dynamic | High, stable, optimized for medical images | Distinguishes between similar densities |
| Uniformity | Can have significant variations | High uniformity across screen (<15-20%) | Consistent image appearance |
| DICOM Compliance | N/A | Essential (e.g., DICOM Part 14 GSDF) | Standardized image presentation |
| Longevity/Stability | Designed for shorter lifespan, less stability | Built for 24/7 operation, long-term stability | Reliability in critical care environments |
These are not minor differences; they are fundamental to the display’s purpose.
Does the cost of modifying consumer-grade displays to meet medical standards offer economic benefits?
Saving money is always appealing. But what if the "savings" end up costing more? Is modifying a cheap screen truly a bargain for hospitals?
The apparent initial cost saving of using a modified consumer display is usually negated by the high costs of necessary upgrades, specialized calibration, and the extensive testing required. Ultimately, it’s often more expensive and less reliable than purchasing a purpose-built medical display.
This is a common misconception I encounter. People see a consumer display for a few hundred dollars and a medical display for several thousand and think there’s a huge margin to play with. However, the cost of genuinely modifying a consumer-grade display to approach medical standards is very high. First, you’d likely need to replace the panel itself with one capable of higher bit depth and better uniformity – if a compatible one even exists. Then, the display’s internal electronics (the controller board, power supply) would need significant upgrades to handle precise calibration, maintain stable luminance, and potentially manage internal sensors for DICOM compliance.
Specialized hardware and software for precise color and grayscale calibration are also significant investments. Each modified unit would need individual, rigorous calibration and ongoing quality assurance. By the time you factor in the engineering time, component costs, calibration, and the necessary testing to even attempt to verify its performance, the final cost can easily exceed that of purchasing a new, certified medical display. From what I’ve seen at Reshin, the "savings" are an illusion. It becomes an uneconomical venture, especially when you consider the lack of warranty and support for such heavily modified units. The hidden costs and risks quickly outweigh any perceived initial benefit.
Can modified consumer-grade displays pass relevant regulatory certifications for medical equipment?
Medical devices face intense scrutiny for safety and effectiveness. Can a souped-up TV really get the official nod for hospital use?
It is highly unlikely that a modified consumer-grade display could pass the stringent regulatory certifications (e.g., FDA 510(k) clearance, CE marking under MDR) required for medical diagnostic equipment. These certifications involve comprehensive testing of safety, performance, and manufacturing quality control, which consumer products are not designed to meet.

This is a major roadblock. Medical displays, like all medical devices, must pass strict industry certifications before they can be legally marketed and used for diagnostic purposes. In the United States, this typically means FDA clearance (like a 510(k) premarket notification). In Europe, it’s the CE mark under the Medical Device Regulation (MDR). These certifications are not just rubber stamps. They involve rigorous testing for electrical safety (e.g., IEC 60601-1), electromagnetic compatibility (EMC, IEC 60601-1-2), biocompatibility of materials if applicable, and critically, proof of clinical performance and consistency.
Consumer-grade displays are simply not designed or manufactured with these standards in mind. The entire design, development, and manufacturing process for a certified medical display like those we produce at Reshin is geared towards meeting these requirements from day one. This includes documented quality management systems (like ISO 13485), risk management, and full traceability of components. Trying to retrofit a consumer product to meet these standards post-hoc is an almost impossible task. Even if you managed some technical upgrades, the lack of documented design controls and manufacturing processes would likely make certification unattainable. Without these certifications, using such a display for medical diagnosis would carry significant legal and patient safety risks.
What is the feasibility of modifying consumer-grade displays into medical displays under current technical conditions?
Even if you ignore cost and rules, can it actually be done well? Are the technical hurdles just too high for a reliable conversion?
Technically, while some parameters might be tweaked, achieving the full suite of medical-grade performance, reliability, and safety features by modifying a consumer display is largely unfeasible. Key aspects like long-term stability, specialized protective designs, and consistent image quality are deeply integrated and not easily added.
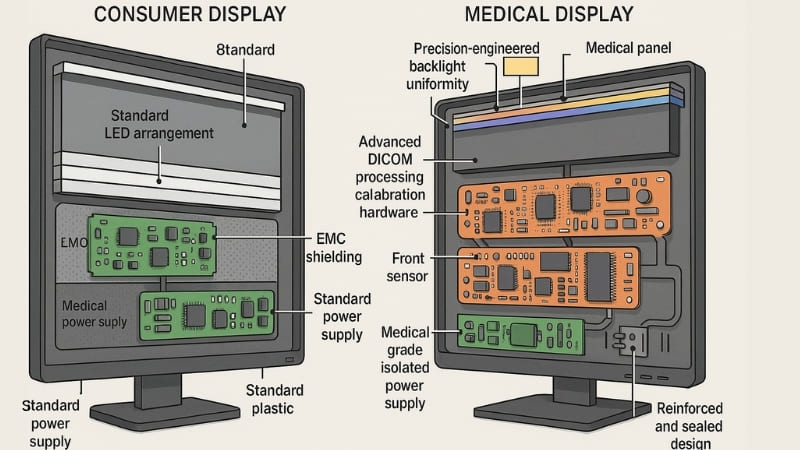
Beyond the specific parameters and certifications, the overall feasibility is very low when you consider the holistic requirements of a medical environment. One critical aspect is service life and stability. Consumer displays are generally built for a shorter operational lifespan and fewer hours of use per day compared to medical displays, which are often run for very long periods, sometimes 24/7, in demanding hospital environments. I’ve seen regular displays exhibit screen aging, image quality degradation (like burn-in or color shift), and brightness loss much faster under such high-load conditions. They simply can’t meet the long-term, high-reliability usage requirements that hospitals depend on.
Furthermore, even if some technical modifications to image parameters were attempted, consumer displays lack crucial medical-grade protective designs. For instance, their electromagnetic compatibility (EMC) is usually not sufficient for a hospital, where many sensitive electronic devices operate. A poorly shielded consumer display could potentially interfere with other critical medical equipment or be susceptible to interference itself. They also typically lack the physical robustness and protection ratings (like IP ratings for dust and liquid ingress resistance) needed to withstand hospital cleaning protocols which often involve harsh disinfectants. Our Reshin displays, for example, have sealed front panels and chemically resistant housings specifically for this reason. These are not features you can easily bolt onto a consumer product; they need to be designed in from the start.
Conclusion
Attempting to modify consumer displays for medical use is a path fraught with technical, financial, and regulatory challenges, ultimately failing to deliver the necessary safety and diagnostic confidence.



