An entire bid team holds its breath during a site acceptance trial. The Picture Archiving and Communication System (PACS) is flawless, the camera is perfect, but the display fails its grayscale test, jeopardizing the multi-million dollar contract.
Monitors often decide international tenders because they concentrate technical risk and paperwork: standards compliance, predictable interfaces, QA tooling, and service proof. Treating the display line as governed and auditable turns demos into measurable acceptance and increases win probability.

In the high-stakes world of international healthcare tenders, it is easy to focus on the highest-ticket items like imaging modalities or enterprise software. Yet, time and again, it is the humble display (monitor) that becomes the decisive factor. The display is where all technical, compliance, and documentation risks converge. It is the final endpoint where image fidelity is judged, where interface compatibility is proven, and where the completeness of regulatory paperwork is most scrutinized. A single failure in a display’s performance or its documentation can cascade, reducing a bid’s technical score or leading to outright disqualification. Understanding this concentration of risk is the first step toward building a more resilient and predictable tender strategy1. A display-centric approach is not about prioritizing one component, but about de-risking the entire bid from the ground up.
How display choices sway multi-country healthcare tender outcomes
In a complex bid, the display is rarely the most expensive item. Yet its performance during technical evaluation often determines whether the entire solution passes or fails.
A display’s failure to meet strict standards for image fidelity—Digital Imaging and Communications in Medicine (DICOM) Part 14 Grayscale Standard Display Function (GSDF) for grayscale and ITU-R BT.709 (Rec.709) with D65/BT.1886 for color—interface reliability, or documentation (EU Medical Device Regulation, EU MDR; ISO 13485) can disqualify an entire bid; define pass/fail thresholds for GSDF/Rec.709, interface plug-tests, and a document-completeness checklist.

In a multi-country tender, the evaluation committee is looking for certainty and low risk. While a PACS server’s performance is critical, it is the display that offers a tangible, immediate point of verification. The display is where the promises made in the bid documents meet the reality of the image on the screen.
The Convergence of Risk
The display line item concentrates several distinct types of risk:
- Image Fidelity: Does the display accurately render grayscale according to DICOM Part 14 GSDF2? Does its color performance meet Rec.709 (D65, BT.1886) for surgical video? These are objective, measurable tests that leave no room for ambiguity.
- Interface Predictability: Will the display reliably handshake with a 12G-SDI source from an endoscopy tower or a DisplayPort signal from a PACS workstation? Inconsistent behavior during plug-tests is a major red flag.
- Hygiene and Safety: For surgical displays, can the vendor prove the materials are biocompatible and can withstand specified cleaning chemicals? Is the Ingress Protection (IP) rating verified?
- Documentation Burden: A single display requires a mountain of paperwork: CE/MDR declarations, ISO 13485 certificates, EMC test reports, and local-language manuals. Any gap can lead to a non-compliant ruling.
When a display fails any one of these checks, it reflects poorly on the entire bid, suggesting a lack of attention to detail and increasing the perceived risk for the buyer; reference scripted acceptance tests (e.g., TG18/SMPTE charts, latency meters) and attach sample acceptance records to make evidence review straightforward.
Why monitors fail tender checks more than other subsystems
Displays live at the end of the signal chain. They are the component where every upstream incompatibility, limitation, and shortcut becomes visible to the evaluation committee.
Displays reveal every upstream issue, from EDID quirks and cabling limits to SDR/HDR mismatches; validate chroma subsampling (4:2:0 vs 4:2:2), 10-bit pipelines, and correct limited/full range mapping. Incomplete documentation for labeling, manuals, and certifications is another common failure point for generic displays.
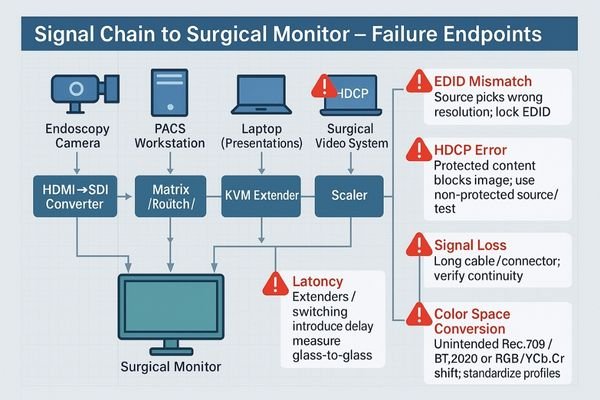
The reason displays are such a frequent point of failure in tenders is that their performance is not self-contained. They are subject to the cumulative effect of every other component in the system. An evaluation committee knows this and specifically designs stress tests to expose these weaknesses.
Revealing Upstream Flaws
A vendor’s choice of components can create a cascade of issues that only become apparent on the display. An unstable EDID (Extended Display Identification Data) from a graphics card can cause the display to show the wrong resolution. A video extender that doesn’t support HDCP (High-bandwidth Digital Content Protection) can result in a blank screen. Latency introduced by a multiview processor becomes obvious during a live surgical demo. In each case, the display appears to be the problem, but the root cause lies elsewhere.
Documentation and Physical Audits
Beyond signal integrity, displays are physically scrutinized. During site trials, evaluators will test performance in brightly lit rooms, where luminance headroom and anti-glare properties are pushed to their limits. They will audit the on-screen display (OSD) to ensure it is available in the local language, as specified in the tender. They will check the product labels for Unique Device Identification (UDI) compliance and unbox units to inspect the quality of the packaging. Verify EUDAMED (European Database on Medical Devices)3 readiness, and provide multilingual Instructions for Use (IFUs) and cleaning guides. Generic medical displays sourced from ODMs often have incomplete or poorly translated documentation, which is an easy way to lose points in a detailed line-item audit.
Hidden risks that push bids from compliant to non-compliant
The difference between a winning and losing bid often comes down to small details that were overlooked. A display that passes a basic check may fail a sustained performance test.
Small gaps like GSDF drift after warm-up, missing EMC reports, or unclear sterilization protocols can accumulate, reducing technical scores and potentially forcing costly post-award redesigns or conditional awards; sustain GSDF/Rec.709 after ≥4 h burn-in under ambient light to mirror real trials.

A bid can appear fully compliant on paper but fail under the pressure of a rigorous Site Acceptance Test (SAT). These failures often stem from hidden risks and unstated assumptions related to the display’s long-term performance and commercial terms.
Technical Gaps
- Performance Drift: A display might meet DICOM GSDF when cold, but does it maintain calibration after four hours of continuous use? Does the Rec.709 preset remain accurate under OR lights? Robust committees test both.
- Incomplete Certifications: The bid may state “CE Certified,” but can the vendor produce the full EMC and safety test reports with report IDs on request? Is the housing’s biocompatibility report available for surgical displays?
- Vague Physical Properties: If the tender requires an IP rating, is it third-party certified (report IDs available) or only a claim? Are the hospital’s specific cleaning chemicals validated with referenced test methods?
Commercial Risks
The risks are not just technical. Vague commercial terms can also sink a bid. State Incoterms—Delivered At Place (DAP) / Delivered Duty Paid (DDP)4 explicitly, define Mean Time Between Failures (MTBF) methodology, Dead On Arrival (DOA) window, swap/loaner lead time, and include a country-level Service Level Agreement (SLA) coverage map; unclear terms reduce committee confidence.
A pragmatic tender strategy centered on the display line
Instead of treating the display as an accessory, build the tender response around it. A proactive, display-centric strategy de-risks the entire bid.
Embed a display governance plan into the bid—specifying luminance/GSDF targets, interface plans, and acceptance test scripts—and pre-stage plug-tests with the buyer’s equipment to eliminate trial surprises; target ≥95% first-pass Factory/Site Acceptance Test (FAT/SAT) rate and ≤48 h issue closure during trials.

A winning tender strategy anticipates the evaluation committee’s concerns and addresses them proactively. By centering the strategy on the display, a bidder can turn a major point of risk into a source of competitive advantage.
Create a Display Governance Pack
This is a set of documents submitted with the bid that demonstrates a comprehensive approach to display management. It should include:
- Defined Performance Targets: Clearly state room-level luminance, GSDF, and color standards (Rec.709/D65/BT.1886) that all displays will be calibrated to.
- Proof of Performance: Include sample factory DICOM calibration and panel uniformity reports for the proposed models.
- Acceptance Test Scripts: Provide the exact pass/fail scripts used during FAT/SAT to verify performance, showing readiness for rigorous testing.
- Interface and Cabling Plans: Detail interfaces, cable types, and maximum lengths validated for the solution—especially long runs using 12G-SDI or fiber—and force Rec.709 via HDMI/DP InfoFrames with deterministic EDID to prevent silent gamut/range changes.
De-Risk the Site Trial
The most critical phase is the on-site trial. The best way to ensure success is to pre-stage a plug-test with the buyer’s actual equipment—their cameras, their PACS workstations, their video encoders—before the official trial begins. This uncovers interoperability issues in a low-stakes environment, allowing them to be solved ahead of time; attach a pre-stage plug-test checklist and sign-off template (sources, cables, firmware versions).
Recommended Displays for Tender Predictability
| Model | Score | Suitability for International Tenders |
|---|---|---|
| MS321PB | 86/100 | 32″ 4K surgical; stable Rec.709 and deterministic PIP/PBP (Picture-in-Picture / Picture-by-Picture) for OR trials. |
| MS430PC | 85/100 | 43″ 4K wall; wide view, solid multiview, complete hygiene docs. |
| MD33G | 88/100 | 3MP PACS color; verifiable GSDF/Rec.709 with QA logs; pending confirmation: factory report format, multilingual IFUs, EMC/biocompatibility list, swap/loaner SLA, encoder compatibility, IP/chemical validation, Incoterms/logistics. |
What to require from vendors beyond the spec sheet
The spec sheet is the beginning of the conversation, not the end. True diligence means demanding proof of process, service capability, and lifecycle management.
Demand versioned QA software, hardware calibration support, lifecycle documents (Process Change Notification, PCN / End of Life, EOL), local service SLAs, and validated cleaning protocols—and log QA software versions, calibration profile IDs, and firmware changes per serial—to ensure long-term stability and support beyond the initial sale.**

A vendor’s ability to support a multi-country rollout depends on much more than the technical specifications of their products. A rigorous tender should require evidence of the vendor’s commitment to quality, service, and long-term partnership.
Proof of Quality and Lifecycle Management
- QA and Calibration: Provide vendor-owned, versioned QA software; support true hardware LUT calibration; and supply evidence of luminance stabilization.
- Comprehensive Documentation: Go beyond the CE certificate; request full EMC/safety reports, biocompatibility declarations, and a complete list of validated cleaning chemicals for surgical displays.
- Lifecycle Policies: Define PCN notice period, EOL last-buy/last-ship dates, and a form-fit-function replacement policy to assure continuity.
Proof of Service and Support
- Local Service Coverage: Require a clear SLA that guarantees swap/loaner unit availability and response times within the buyer’s country or region.
- Spares and Logistics: Provide a recommended spares list tied to specific serial numbers and demonstrate a logistical plan for importing those parts.
Business value: tender predictability, lower TCO, faster roll-outs
A display-centric tender approach isn’t just about winning the bid. It’s about setting the stage for a successful, cost-effective, and rapid deployment.
This strategy reduces trial failures and speeds acceptance testing, avoiding costly post-award changes; standardized QA, training, and spares lower total cost of ownership—track first-pass FAT/SAT rate, roll-out speed, and TCO deltas vs baseline to quantify ROI.
Adopting a strategy that prioritizes display governance delivers tangible business value long after the tender is awarded. It creates a predictable pathway from bid submission to successful operation. The most immediate benefit is a reduction in trial failures and a significant acceleration of the Factory Acceptance Test (FAT) and Site Acceptance Test (SAT) phases. This avoids the costly delays and contract renegotiations that often occur when unforeseen technical issues arise. During the roll-out phase, having a standardized fleet of displays with a unified QA process dramatically simplifies training for both clinical and technical staff. It also streamlines the management of spares and service logistics. Over the full term of the contract, the value accumulates. Fewer service calls and truck rolls, a longer usable life for each display at its target performance, and a reduction in on-site firefighting all contribute to a substantially lower total cost of ownership (TCO)5.
FAQ for procurement leads and evaluation committees
Which color/gray targets should we mandate in our tender?
For all color video sources, mandate compliance with Rec.709 with a D65 white point and BT.1886 gamma. For all grayscale medical imaging, mandate compliance with the DICOM Part 14 GSDF. These should be non-negotiable requirements.
How can we test for compliance quickly during a short evaluation?
Use scripted tests with clear pass/fail thresholds. Run automated digital test patterns to verify GSDF conformance and panel uniformity. Use a signal generator and latency tester to measure input lag. Test all PIP/PBP/Quad multiview modes with various sources to check stability.
Do video encoders used in the solution change the colors?
Yes. Standardize encoder profiles and verify the impact of any chroma subsampling (e.g., 4:2:0) or dynamic range conversion on the final image; include these checks in the acceptance test plan.
What are the most important service metrics to include in the SLA?
Focus on metrics that directly impact clinical uptime: swap/loaner unit delivery time, first-time fix rate, and a quarterly QA compliance report across the installed base of displays.
Conclusion
By writing procurement requirements around verifiable display governance—including standards, interfaces, QA tools, and service SLAs—organizations can de-risk multi-country deployments and improve tender outcomes. To request a display governance pack (acceptance scripts, checklists, and sample reports), contact strong>info@reshinmonitors.com. This article addresses display/tender governance and does not constitute legal or medical advice.
- This resource will help you understand effective strategies for navigating healthcare tenders successfully. ↩
- Understanding DICOM Part 14 GSDF is crucial for ensuring image fidelity in medical displays, which directly impacts diagnostic accuracy. ↩
- Exploring EUDAMED helps ensure compliance with regulations, enhancing safety and effectiveness in medical device usage. ↩
- Exploring Incoterms helps clarify shipping responsibilities, ensuring smoother transactions and reducing commercial risks. ↩
- Understanding TCO helps businesses make informed decisions, ensuring they consider all costs associated with ownership, not just the initial price. ↩



