Choosing materials for medical devices is crucial. Industrial or medical-grade – what’s the real difference? This uncertainty affects safety concerns in critical care. Understanding this distinction highlights the quality designed into specialized medical equipment.
Medical-grade materials undergo rigorous biocompatibility testing, ensuring patient safety and sterilization endurance. Industrial materials, robust yet focused on mechanical performance and cost, lack the strict safety and regulatory scrutiny essential for clinical use. This gives medical-grade components superior safety, chemical resistance, and specialized healthcare properties.
This critical difference impacts device safety, longevity, and overall compliance. Let’s explore what truly sets these material grades apart.
What defines a material as "medical-grade"?
It’s tempting to think any strong material suffices. But in medicine, the definition is far more complex, and overlooking this can lead to serious issues for patient well-being.
A "medical-grade" material is proven biocompatible, meets strict regulatory standards like ISO 10993, and is suited for its intended medical use, often involving patient contact, ensuring it won’t cause harm.

When we speak of "medical-grade," we refer to materials specifically designed, manufactured, and stringently tested for healthcare. This isn’t merely a label but a comprehensive qualification. The cornerstone of a medical-grade material is its biocompatibility1. This means it has been thoroughly evaluated to ensure it doesn’t cause adverse reactions—like toxicity, irritation, or allergic responses—when in contact with human tissues or fluids. The international standard ISO 10993, "Biological evaluation of medical devices," provides the framework for these crucial tests. Industrial materials, focusing mainly on mechanical performance, don’t typically undergo such biological safety assessments.
Furthermore, medical-grade materials must often demonstrate:
- Sterilizability2: The capacity to endure common sterilization methods (e.g., autoclaving, gamma irradiation) without degradation.
- Chemical Resistance3: Resilience against the harsh disinfectants frequently used in clinical settings.
- Traceability and Consistency: Detailed records of origin, composition, and batch-to-batch consistency are vital.
These attributes are fundamental. For instance, when developing our Reshin surgical displays, such as the MS270P 27" FHD Surgical Display, the materials for casings and screens are selected based on these medical-grade criteria, as they will be present in sterile or near-sterile environments. Industrial materials are usually chosen for mechanical strength or cost in applications like automotive parts, not for direct or indirect patient interaction.
How do safety and compliance standards differ between industrial and medical use?
One might assume safety standards are universally strict. However, regulations for medical devices are exceptionally rigorous, and confusing industrial compliance with medical suitability can be a critical oversight.
Medical material safety involves patient-centric standards like FDA regulations, CE marking (MDR), and ISO 13485. Industrial standards (e.g., RoHS) address environmental or occupational safety, not direct patient interaction.

The divergence in safety and compliance standards between industrial and medical materials is substantial, directly reflecting the potential impact medical devices have on patient health.
Medical-Grade Standards & Compliance4:
These are profoundly stringent, prioritizing patient risk minimization. Key frameworks include:
- Regulatory Bodies: Authorities like the FDA in the USA (under CFR Title 21) and European regulations (CE marking under MDR/IVDR) impose strict controls. This involves premarket approvals or clearances that intensely scrutinize material safety.
- Biocompatibility Standards5: ISO 10993 is paramount, detailing biological evaluation tests for cytotoxicity, sensitization, irritation, and systemic toxicity to ensure materials are safe for human contact.
- Quality Management Systems6: ISO 13485 dictates quality management for medical device manufacturing, where material sourcing, control, and traceability are critical.
- Risk Management: ISO 14971 outlines processes for identifying and mitigating risks associated with medical devices, including those posed by materials.
Industrial-Grade Standards & Compliance:
These standards, while important, address different concerns:
- Environmental & Occupational Safety: Standards like RoHS (Restriction of Hazardous Substances) and REACH (Registration, Evaluation, Authorisation and Restriction of Chemicals) focus on limiting hazardous materials in products or ensuring chemical safety for workers and the environment, not direct patient biocompatibility.
- Performance Metrics: ASTM or ISO standards might define tensile strength, impact resistance, or UV stability, crucial for industrial performance but not inherently for patient safety in a clinical context.
At Reshin, when engineering products like the MS321PB 32" 4K Surgical Monitor, adherence to these demanding medical standards for all patient-contact or near-patient components is integral. This commitment to superior compliance and safety is why our brand is trusted globally by leading names and in countless hospitals.
Why are biocompatibility and sterilizability critical in medical materials?
Imagine a device causing an allergic reaction or harboring bacteria in surgery. These aren’t minor issues; they pose severe threats within any healthcare environment, especially sterile fields.
Biocompatibility prevents adverse patient reactions (toxic, allergic). Sterilizability ensures materials withstand repeated sterilization, vital for infection prevention and maintaining a safe surgical environment for everyone involved.
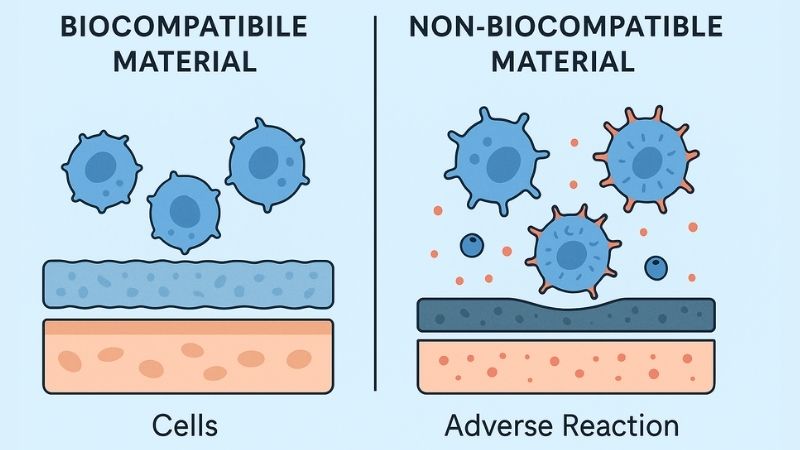
In medical settings, especially operating rooms or for implantable devices, biocompatibility7 and sterilizability8 are non-negotiable for ensuring patient safety and successful clinical outcomes.
Understanding Biocompatibility:
Biocompatibility signifies a material’s ability to perform its intended function without provoking undesirable local or systemic effects in the patient. The material must harmoniously coexist with biological systems. A non-biocompatible material might:
- Induce cytotoxicity, harming or killing cells.
- Cause sensitization, leading to allergic reactions.
- Result in irritation or inflammation at the contact site.
- Exhibit systemic toxicity, affecting the entire body.
- Pose carcinogenic risks.
Extensive testing under ISO 109939 assesses these potential harms. For components of our Reshin surgical displays, such as the MS430PC 43" 4K Surgical Monitor, which are close to the surgical field or touched by staff, ensuring biocompatible casings and screens is paramount.
The Importance of Sterilizability:
Medical devices, particularly those used in surgical or invasive procedures, must be sterile to prevent healthcare-associated infections (HAIs). Materials in these devices must therefore endure common sterilization methods—such as autoclaving (steam), ethylene oxide (EtO) gas, gamma irradiation, or hydrogen peroxide plasma—without degrading. The material must maintain its critical properties (strength, clarity, color) and appearance through multiple cycles if it’s reusable. If a material warps, becomes brittle, or leaches chemicals post-sterilization, it’s unsuitable. This is a core design principle for Reshin monitors, built to withstand rigorous hospital cleaning protocols.
How do durability and chemical resistance compare between the two grades?
An office monitor might face an occasional spill. A surgical display, however, endures daily onslaughts of harsh cleaning agents, demanding an entirely different level of material resilience.
Medical-grade materials are engineered for repeated exposure to potent disinfectants and demanding clinical use, showing superior chemical resistance. Industrial materials focus on mechanical or environmental wear, not hospital-grade cleaners.

While durability is a goal for both industrial and medical-grade materials10, the specific nature of that durability, especially concerning chemical resistance, often varies dramatically.
Industrial Material Durability & Chemical Resistance:
Industrial materials are chosen for robustness against specific operational stresses—this might be mechanical loads, abrasion, UV exposure, or resistance to industrial chemicals like oils and solvents. However, they are typically not formulated to withstand the unique and aggressive chemical environment found in hospitals. Common plastics used in industrial products can crack, cloud, become brittle, or leach harmful substances when repeatedly exposed to the potent disinfectants (e.g., quaternary ammonium compounds, bleach, concentrated alcohol solutions) necessary to maintain sterility and hygiene in healthcare settings. Regular disinfection with harsh chemicals can degrade these industrial materials11 quickly.
Medical-Grade Material Durability & Chemical Resistance:
Medical-grade materials, especially those forming device housings like our Reshin surgical displays (e.g., the MS550P 55" 4K Surgical Monitor), must exhibit exceptional chemical resistance. They are specifically selected or formulated to:
- Resist Chemical Erosion: Maintain structural integrity, appearance, and functionality after numerous cleanings with a broad spectrum of hospital-grade disinfectants, preventing issues like cracking, crazing, or discoloration.
- Prevent Leaching: Ensure no harmful substances are extracted by cleaning agents, which could then pose a risk to patients or users.
- Maintain Surface Properties: Critical features such as anti-glare coatings, antimicrobial properties, and waterproofing on displays and interfaces must remain effective. Industrial materials typically lack these specific enhancements. For example, our monitors, from the MS192SA 19" HD Endoscopic Monitor to larger 4K models, feature robust front surfaces designed for such resilience.
This enhanced chemical resistance12 often comes from using specialized polymers (e.g., medical-grade polycarbonates, ABS/PC blends, polysulfones) and advanced surface treatments, ensuring longevity in challenging clinical environments.
Why does Reshin use medical-grade materials in its surgical displays?
When a surgeon relies on our display for critical intraoperative imaging, material failure or safety hazards are unacceptable. Our material choices directly reflect our commitment to excellence and safety.
Reshin uses medical-grade materials for casings, coatings, and contact surfaces in surgical displays. This ensures patient/user safety, device longevity, and reliable performance despite frequent, harsh cleaning and intensive OR use.
At Reshin, our dedication to advancing medical display technology means providing high-quality, exceptionally reliable solutions for medical professionals. A fundamental aspect of this is our unwavering commitment to using medical-grade materials in constructing our surgical displays, from the compact MS247SA 24" FHD Endoscopic Monitor to the advanced MS275P 27" 4K Surgical Monitor. This choice is driven by several critical factors:
-
Upholding Patient and User Safety: This is our absolute priority. Surgical environments operate under the strictest hygiene and safety standards. By employing materials that are rigorously tested for biocompatibility1 and are resistant to microbial contamination (or are designed for easy, thorough sterilization), we significantly minimize risks of adverse reactions and healthcare-associated infections. This safeguards both the patient and the clinical team.
-
Ensuring Device Longevity and Unwavering Reliability: Operating rooms represent demanding environments. Equipment is constantly handled, repositioned, and, most importantly, subjected to stringent cleaning and disinfection. Medical-grade materials are specifically selected for their superior ability to withstand harsh chemical agents and repeated sterilization cycles without degrading. This means our monitors, including the robust MS321PC 32" 4K Surgical Monitor, maintain their structural and functional integrity over an extended service life, offering better long-term value.
-
Maintaining Optimal Performance Integrity: The visual performance of a surgical display is paramount. Medical-grade screen coatings, including anti-glare and anti-reflective layers, must be exceptionally durable to withstand cleaning without compromising image clarity, color accuracy, or brightness. Industrial coatings might wear quickly or react negatively with disinfectants, impairing the very function of the display.
-
Adherence to Global Regulatory Standards: Utilizing certified medical-grade materials is key to ensuring our products meet the stringent requirements of health authorities globally (e.g., FDA, CE MDR). This is vital for market access and underscores our commitment to quality, an important consideration for healthcare institutions during their procurement processes. Our global track record, including collaborations with esteemed brands, further attests to this dedication.
By investing in superior medical-grade materials, Reshin ensures its surgical displays2 are not only technologically advanced but also fundamentally safe, durable, and perfectly suited for the critical demands of modern medical practice.
Conclusion
The distinction is vital: medical-grade materials offer superior safety, chemical resilience, and regulatory adherence crucial for healthcare. Industrial materials, prioritizing mechanical aspects and cost, fall short in clinical settings. For monitors built with certified medical-grade materials, contact Reshin at martin@reshinmonitors.com.
-
Understanding biocompatibility is crucial for ensuring safety in medical applications. Explore this link to learn more about its importance. ↩ ↩
-
Sterilizability is essential for maintaining hygiene in healthcare. Discover more about its significance in medical materials. ↩ ↩
-
Chemical resistance ensures durability and safety in clinical settings. Learn more about its role in medical materials. ↩
-
Understanding these standards is crucial for ensuring patient safety and compliance in medical device manufacturing. ↩
-
Exploring biocompatibility standards helps ensure that materials used in medical devices are safe for human contact, which is vital for patient health. ↩
-
Learning about quality management systems can enhance your understanding of how they ensure safety and compliance in medical device production. ↩
-
Understanding biocompatibility is crucial for ensuring patient safety and effective medical device performance. Explore this link to learn more. ↩
-
Sterilizability is vital to prevent infections in medical settings. Discover why it matters for patient safety and device reliability. ↩
-
ISO 10993 is essential for assessing biocompatibility. Learn about its standards and how they ensure safe medical materials. ↩
-
Discover the advantages of medical-grade materials, which are essential for maintaining safety and effectiveness in healthcare settings. ↩
-
Learn about the key differences between industrial and medical-grade materials, especially regarding their chemical resistance and durability. ↩
-
Explore this link to understand the best materials that ensure safety and durability in medical environments, crucial for patient care. ↩



