Ophthalmic procedures create some of the most demanding surgical monitor requirements because visibility is stressed by extreme coaxial illumination, specular reflections, and ultra-fine anatomical detail that must remain readable across long cases. In real deployments, problems are more often caused by highlight clipping, dynamic processing drift, scaling artifacts, or latency variability than by any single “panel spec” on a datasheet.
Selecting surgical monitors for ophthalmic procedures requires matching display behavior to segment-specific visibility risks: smooth highlight handling and stable brightness for anterior segment work, predictable color and tonal mapping with stable low-level detail for posterior segment procedures. Prioritize clean scaling, consistent low latency, and validated picture modes that prevent drift during procedures.
Successful monitor selection1 depends on mapping the display’s behavior to the segment workflow and validating the complete signal chain that will be used for surgery, recording, and teaching. This approach emphasizes repeatability—meaning the same delivered signal looks consistent across switching, rebooting, recording on/off states, and shared viewing—rather than relying on marketing labels that may not reflect real clinical conditions.
What makes ophthalmic surgical video viewing different for anterior vs posterior segments?
Ophthalmic procedures place unusually high demands on monitor performance due to extreme illumination and fine tissue detail requirements.
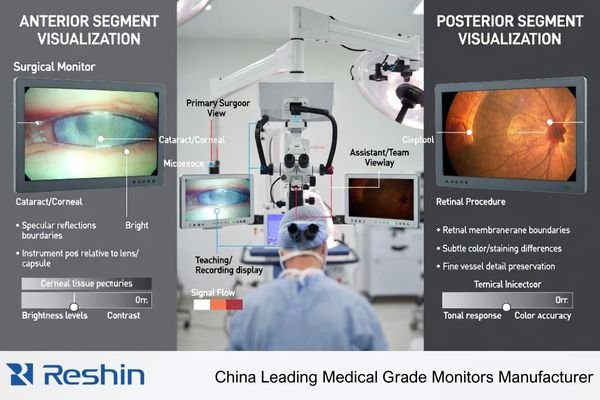
Ophthalmic procedures demand exceptional perceived sharpness, color neutrality, and stable visibility because surgeons rely on fine tissue boundaries, subtle transparency changes, and specular highlights under intense coaxial illumination. Anterior segment work involves bright fields and delicate contrast in translucent tissues requiring smooth highlight handling, while posterior segment workflows depend on consistent color rendering and contrast in darker, lower-signal scenes where poor black handling can mask clinical detail.
Monitor selection should reflect that “good looking” in a demo is not the same as reliable visibility in a case. The key is to avoid processing that manufactures apparent sharpness (halos, ringing, over-sharpening) while degrading real micro-structure. Because the same monitor may also serve assistants, scrub nurses, recording, and teaching views, consistency across viewing angles and system states matters as much as peak clarity.
Anterior Segment Requirements
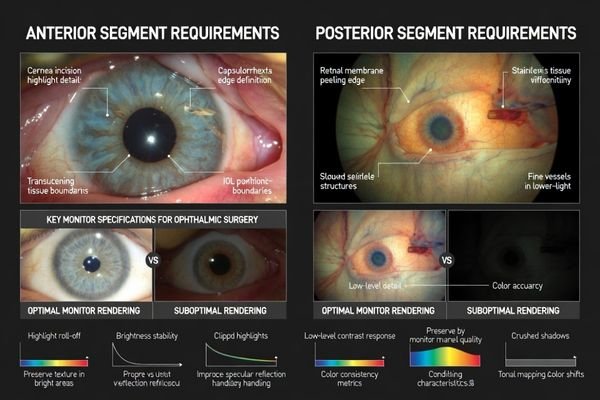
Anterior segment cases frequently involve bright fields, reflections, and delicate contrast in translucent tissues, so highlight roll-off2 becomes a primary risk. A suitable monitor preserves texture in bright areas without clipping to flat white and maintains micro-contrast without artificial edge halos around specular reflections that can distort perceived boundaries. Stable brightness under intense illumination helps keep the view predictable throughout long cases.
Posterior Segment Challenges
Posterior segment workflows depend on stable low-level detail and predictable tonal/color behavior in darker, lower-signal scenes that include retinal detail, membranes, and subtle staining cues. If black handling is poor or dynamic processing shifts during the procedure, fine membrane edges and faint cues can be masked, and trust drops when switching between live view, playback, and shared displays. The goal is a believable, repeatable image that doesn’t drift as conditions change.
How do resolution, scaling, and latency influence ophthalmic precision and comfort?
Resolution scaling and latency management directly impact surgical precision and team coordination in ophthalmic procedures.
Ophthalmic video magnifies small structures to resolution limits, making scaling mismatches between source output and panel resolution critical because they introduce softness, ringing, or edge halos that distort perceived boundaries and depth cues. In anterior segment cases with moving instruments and reflective surfaces, consistent latency across input switching and long sessions affects hand-eye coordination more than absolute latency values, requiring stable signal processing.
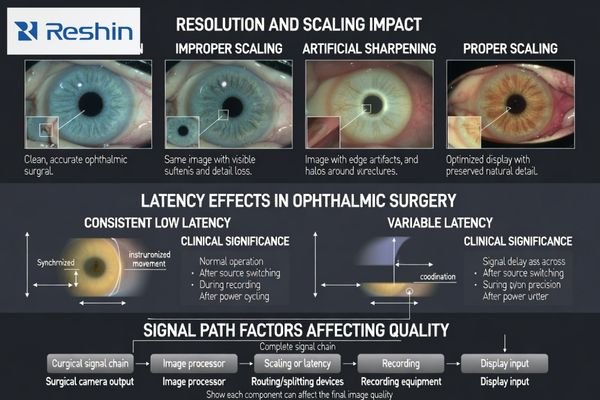
Many OR chains include splitters, converters, capture devices, or recorders. Each stage can resample the signal (changing perceived sharpness) or buffer it (changing delay), and the combined behavior is what the surgeon experiences. This is why “camera label” and “monitor label” are less useful than verifying the delivered format and the resulting on-screen behavior.
A reliable selection approach is to confirm the actual delivered format from the surgical system and routing path, select a monitor that handles that format cleanly with minimal enhancement, and validate latency in the real workflow. For ophthalmic work, consistent latency3 across switching, recording enable/disable, and long sessions is often more important than a single nominal latency number.
What brightness, highlight, and color controls matter most under ophthalmic illumination?
Ophthalmic illumination creates extreme highlight conditions that require specific monitor control capabilities.
Ophthalmic illumination creates extreme highlight conditions including specular reflections, bright corneal surfaces, and rapid exposure changes that challenge monitors with poor highlight roll-off or aggressive dynamic contrast. Anterior work requires smooth highlight handling preventing clipped whites, while posterior work needs stable low-level luminance and predictable tonal mapping. Color should be consistent and controllable rather than enhanced, with ability to lock validated picture modes.
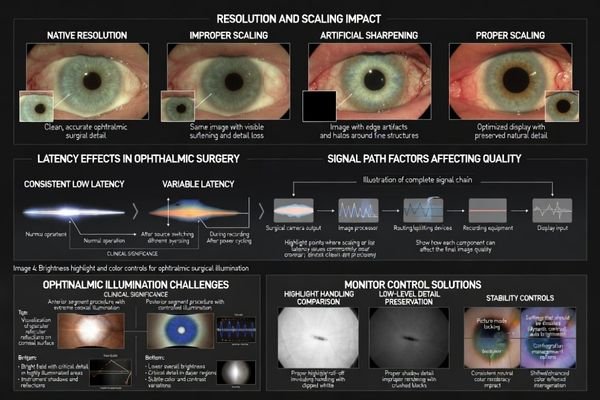
When ophthalmic installations experience visibility issues, the cause is often uncontrolled processing—dynamic contrast, “auto” enhancement, or modes that change with content—rather than a lack of raw resolution. That drift reduces confidence when switching between live viewing, recorded playback, and shared team displays.
| Control Feature | Anterior Segment Priority | Posterior Segment Priority | Clinical Impact | Team Communication Effect |
|---|---|---|---|---|
| Highlight Handling | Critical for specular reflections | Moderate importance | Preserves texture in bright areas | Consistent reflection interpretation |
| Low-Level Detail | Secondary concern | Critical for membrane visibility | Reveals subtle clinical cues | Uniform shadow detail perception |
| Color Consistency | Important for transparency cues | Critical for staining visibility | Maintains diagnostic confidence | Reliable documentation matching |
| Dynamic Processing | Disable to prevent clipping | Disable to prevent crushing | Avoids masking clinical detail | Prevents perception drift |
| Picture Mode Lock | Essential for stability | Essential for repeatability | Maintains validated response | Ensures team alignment |
For anterior segment procedures, smooth highlight handling4 prevents clipped whites that erase texture and makes reflections easier to interpret without overwhelming surrounding detail. Stable brightness under intense illumination helps maintain a consistent adaptation state for the team throughout the case.
Posterior segment workflows benefit from stable low-level luminance and predictable tonal mapping that preserve subtle membrane edges and staining cues without crushing shadows or introducing dynamic contrast behaviors that drift during extended procedures.
Practical controls supporting repeatability include locking validated picture modes, disabling dynamic enhancements, and maintaining uniform appearance so teams see identical cues across screens and throughout surgical cases.
How should you validate the end-to-end signal chain for ophthalmic surgery?
Validation should treat surgical cameras, processing, routing, and displays as integrated systems rather than individual components.
Validation should treat surgical camera systems, image processors, routing devices, capture equipment, and monitors as single systems because common failures result from component interactions. Document exact output modes, verify monitor scaling and stability, test state changes including source switching and power cycling, confirm live view and playback consistency, and record baseline configurations preventing silent drift from updates or replacements.
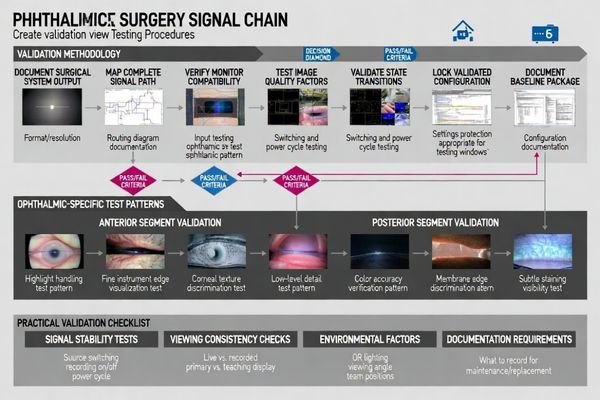
Systematic validation should focus on what will actually happen during cases: switching sources, enabling/disabling recording, reconnecting cables, and recovering from power cycles. Small state-dependent changes can be disruptive in ophthalmic workflows, so the acceptance goal is repeatability across those real transitions—not a one-time “looks good” check.
System Integration Testing5
Start by documenting the exact output modes delivered to monitors in intended configurations, then verify that monitors display signals with correct scaling, stable brightness behavior, and minimal processing artifacts that could affect clinical interpretation. Confirm that picture mode settings can be locked and that “auto” enhancements can be disabled so the validated view can be reproduced reliably across cases.
State Change Validation
Test practical state transitions including source switching, power cycling, cable reconnection, and recording enable/disable because ophthalmic workflows often switch rapidly between views and teaching modes. Verify that the monitor returns to the validated mode after each transition and that any live view vs. playback differences remain acceptable for your documentation and teaching use case.
Selecting surgical monitors for anterior and posterior segment ophthalmic procedures
Monitor selection should begin with procedure types and actual video pipelines rather than generic specifications.
Effective selection strategies work backward from clinical workflows and delivered signal characteristics.
Choose ophthalmic surgical monitors by starting with procedure types and real video pipelines, then matching display behavior to segment visibility risks rather than pursuing headline specifications.
| Clinical Role / Application | Usage Pattern | Display Requirements | Recommended Model | Key Integration Considerations |
|---|---|---|---|---|
| Anterior Segment Primary | High-magnification precision work | Smooth highlights, minimal latency | MS270P | Direct signal path, locked picture modes |
| Posterior Segment Focus | Fine retinal detail visualization | Stable low-level detail, predictable color/tones | MS275PA | Consistent settings, repeatable brightness |
| Multi-Segment Flexibility | Anterior and posterior procedures | Balanced highlight and shadow handling | MS321PB | Routing validation, mode stability |
| Advanced Visualization | Complex surgical documentation | Stable processing, clean scaling | MS321PC | Validate on real feed, consistent state behavior |
| Team Coordination Display | Shared surgical visualization | Large format, consistent appearance | MS322PB | Viewing angles, uniformity, repeatability |
For anterior and posterior segment workflows, start by mapping the monitor’s role (primary surgeon view, assistant view, shared display, teaching/recording) to the real video pipeline and viewing distance, then prioritize stable image behavior over “enhanced” presentation. For anterior cases, focus on smooth highlight handling and stable brightness under intense illumination to avoid clipped whites and reflection-driven artifacts; for posterior cases, focus on predictable tonal/color mapping and stable low-level detail so subtle membrane edges and staining cues remain visible without dynamic drift. In both, validate the delivered input format through your actual routing (splitters/converters/capture/recording), choose clean scaling with consistent low latency, lock the validated picture mode, and factor in OR constraints such as arm mounting, cable strain relief, cleanable sealed surfaces, and replacement consistency to prevent re-validation delays.
FAQ
Do anterior segment procedures require different monitor settings than posterior segment procedures?
Often yes; anterior work is more sensitive to highlight handling and reflections, while posterior work is more sensitive to low-level detail and predictable tonal/color mapping, so validated modes should match the segment workflow.
Is a "4K" label enough to guarantee better ophthalmic visibility?
No; the delivered output format, scaling behavior, and processing controls matter more than labels, and should be verified with your actual camera and routing chain.
How can I tell if the monitor is clipping highlights in anterior segment views?
Watch specular reflections and bright corneal surfaces for "flat white" areas with lost texture, and compare with dynamic enhancements disabled in a locked mode.
What latency risks matter most in ophthalmic workflows?
Variable latency across switching, recording, or routing changes is often more disruptive than a single static number; test the full chain in the real workflow.
Do recording and teaching feeds change what the surgeon sees?
They can; buffering and resampling may alter sharpness and delay, so validate that live view and playback remain acceptably consistent for your use case.
What should be documented to avoid re-validation delays later?
Document the delivered output mode, the routing diagram, the locked picture mode/settings, and the acceptance checks for scaling, stability, and latency.
Conclusion
Selecting surgical monitors for ophthalmic anterior and posterior segment procedures focuses on achieving repeatable visibility under extreme illumination and fine-detail demands rather than pursuing panel specifications alone. The most effective selection process prioritizes smooth highlight handling and stable brightness for anterior segment workflows, while emphasizing predictable tonal and color behavior with stable low-level detail for posterior segment applications. Success also depends on clean scaling and consistent latency through the complete signal chain that includes routing, conversion, and recording components.
Our methodology at Reshin emphasizes validating complete systems from surgical cameras through all signal processing stages to final display, followed by locking and documenting baseline configurations to prevent switching, resets, or replacements from introducing silent drift that compromises clinical visibility. With segment-matched settings and controlled workflows, surgical monitors become reliable extensions of visualization systems that support both surgical precision and effective team communication throughout demanding ophthalmic procedures.
✉️ info@reshinmonitors.com
🌐 https://reshinmonitors.com/
-
Understanding best practices for monitor selection can enhance surgical outcomes and ensure consistency in clinical settings. ↩
-
Understanding highlight roll-off is crucial for ensuring accurate image representation in medical monitors, especially in delicate procedures. ↩
-
Consistent latency is vital in ophthalmic procedures to maintain precision and reliability during long sessions, ensuring patient safety. ↩
-
Explore how highlight handling preserves texture and improves interpretation in clinical imaging. ↩
-
Understanding System Integration Testing is crucial for ensuring reliable monitor performance in clinical settings. ↩