Specification Baseline
Define a clear specification baseline and configuration scope for OEM medical grade monitors programs, aligned with integration and manufacturing expectations.
Reshin provides OEM/ODM customized medical display solutions optimized for integration into surgical, endoscopic, and diagnostic systems. Our manufacturing-driven customization process focuses on repeatability, controlled configuration, and documentation discipline, reducing integration risk and enabling predictable volume supply.
Customized projects require medical grade monitors to remain stable after validation. Reshin evaluates customization requests for manufacturability, supply stability, and documentation impact before committing to long-term production.
Structured OEM manufacturing programs built for repeatable quality, stable scheduling, and reliable delivery across long product lifecycles.
Controlled configurations with documented change control to keep BOM consistency, reduce variation, and support multi-batch supply.
Pilot runs before volume production to validate performance, manufacturability, and packaging/labeling details with fewer surprises later.
Engineering cooperation for integration, covering I/O planning, mounting/fit checks, and system-level considerations for deployment.
Documentation support for compliance needs, including model-based document availability guidance for procurement review and audits.
Long-term supply mindset for deployed systems, prioritizing continuity, consistency, and practical support for fielded installations.
OEM medical-grade monitor programs for endoscopy, surgical imaging, and diagnostic system platforms.
Integration-ready medical-grade monitors with stable configuration behavior for deployment and workflow fit.
Manufacturer-level cooperation for stable supply, technical coordination, and responsive project support.
Customization in medical grade monitors is not about changing specifications. For OEM teams, customization must be evaluated as a manufacturing program—focused on repeatability, integration behavior, and controlled changes after validation.
Repeatability: consistent configuration from sample to volume
Change control: validated parameters are frozen and managed under documented process
Documentation impact: customization choices align with compliance documentation needs
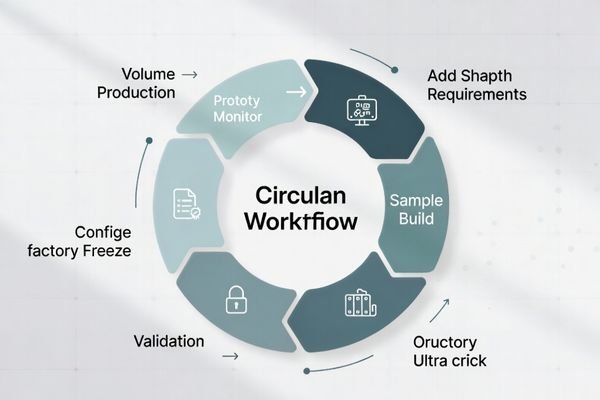
All customization options are evaluated under manufacturing, integration, and compliance frameworks.Customized medical display solutions for surgical 4K UHD, DICOM diagnostic displays, and multi-input PiP/PBP OR monitors are built to fit workflows with OEM/ODM reliability and compliance.
19″–55″ options align with OR, PACS, and ICU layouts, reducing retrofit effort and on-site time.
FHD / 2–12MP / 4K UHD selections match modality needs, balancing clinical accuracy and budget control.
HDMI/DP/12G-SDI/DVI/VGA enable stable multi-input PiP/PBP, simplifying hybrid-OR integration and reducing adapter risks.
AR glass, optical bonding, sealing, and IP-Cap touch improve durability and support infection-control compliance.
Black/white shells, slim bezels, and branding options align with hospital identity while avoiding supply complexity.
VESA wall, arms, carts, and stands ensure quick fit to existing hardware and integration plans.
IP sealing, antimicrobial coatings, and IEC/ISO compliance help reduce operational risk at demanding sites.
Custom layouts and cable routing fit installation paths, cutting on-site time and rework.
300–1000 nits with HDR and CBS stabilization are maintained to keep image clarity through full shifts and support consistent reads.
DDICOM GSDF/LUT with auto-calibration is recorded, keeping QA predictable and audit-ready for PACS workflows.
Preset gamma for CT, MRI, angiography, endoscopy, and mammography shortens setup and reduces user variance across sites.
Fanless/silent cooling and efficient design keep OEM medical monitors quiet, reliable, and easier to maintain.
Low-blue, flicker-free, and auto-brightness modes help reduce fatigue during long diagnostic reads.
Backlight control, luminance stabilization, and uniformity correction are applied to cut rereads and field variance.
PIP/PBP/triple/quad views enable side-by-side comparison without extra workstations, simplifying OR and radiology workflows.
BT.2020 color with DICOM grayscale keeps visualization accurate across modalities and sites.
12/24V DC, AC, or dual-redundant power is supported to sustain safety during critical procedures.
IEC 60601-1, DICOM Part 14, and CE/FDA readiness are documented to accelerate market entry and audits.
Fleet diagnostics, firmware status, and QA logs are supported to simplify multi-site IT management and service response.
Internal storage retains calibration and QA history, keeping compliance traceable and reducing review time.
Multi-language OSD (EN/中文/FR/AR…) supports international rollouts and distributor training.
Telemedicine, teaching, and pathology are supported with tailored workflows and documentation.
PACS/HIS/RIS connectivity standardizes hospital workflows and lowers adaptation cost for integrators.
Some OEM programs for medical grade monitors require local assembly or customer-owned branding. The table below clarifies what each supply model means and where responsibility typically sits, helping procurement and product teams align expectations early.
| Supply Model | What You Receive | Who Completes Final Steps | Best Fit |
|---|---|---|---|
| OEM (Fully Assembled) | Finished medical grade monitors built to the agreed configuration. | Reshin completes assembly and labeling per the approved spec. | Standard OEM deployments requiring ready-to-install supply. |
| SKD (Semi-Knocked Down) | Kit or semi-assembled set with defined scope and parts list. | Customer or local partner completes final assembly and/or labeling within an agreed boundary. | Local assembly requirement, logistics/tariff strategy, or regional program constraints. |
| Customer-Brand Manufacturing | Finished unit manufactured under customer-owned branding rules. | Branding/labeling follows customer ownership and documentation rules. | Customer portfolio branding with stable documentation alignment across markets. |
| ODM (Optional) | Co-defined platform with deeper design definition under program scope. | Typically manufacturer-led build with verification and controlled changes. | Programs needing deeper design changes and formal change-control discipline. |
These customization programs were executed as controlled manufacturing projects, many of which entered pilot and volume production stages.
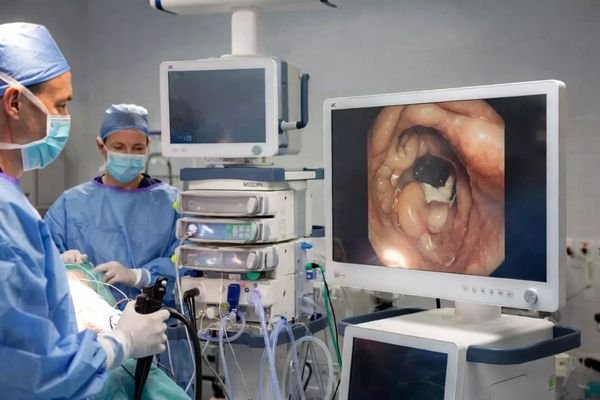
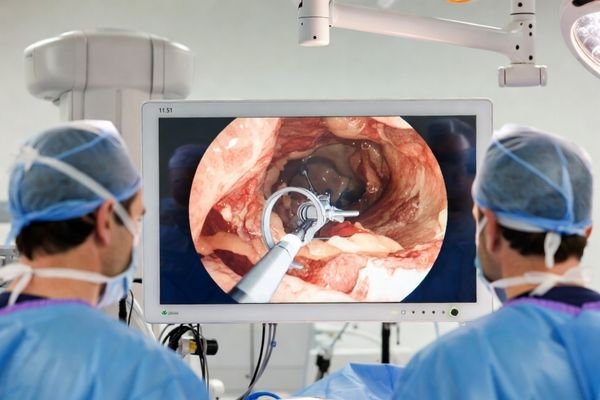
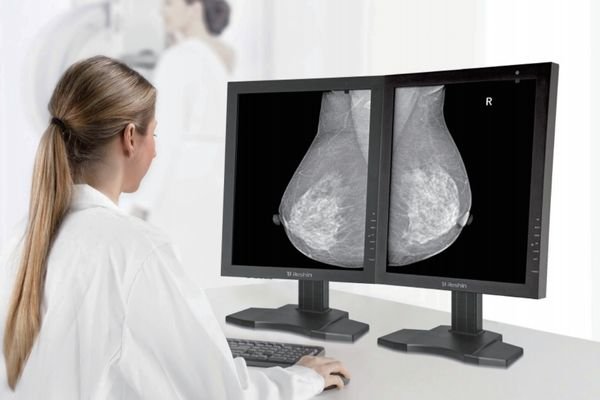
Select size, resolution, interfaces, brightness, and protection level online. A tailored customized medical display solution with BOM support is returned within one business day, making evaluations faster.
Customization and OEM programs for medical grade monitors often affect documentation scope and local registration workflows. We support OEM teams with structured documentation coordination and engineering cooperation—focused on configuration definition, repeatability, and clear responsibility boundaries.
Define a clear specification baseline and configuration scope for OEM medical grade monitors programs, aligned with integration and manufacturing expectations.
Coordinate labeling and identification inputs to match customer program requirements and target-market expectations, with clear responsibilities.
Provide manufacturing and traceability coordination information suitable for OEM workflows and internal QA, consistent with production control.
Support customer internal reviews with verification summary inputs aligned to configuration freeze and repeatable manufacturing expectations.
Provide a practical checklist describing what can be prepared for the specific medical grade monitors program and what remains customer-led.
Local registration is led by the medical system manufacturer or authorized representative. We provide technical documentation coordination and engineering cooperation; we do not make registration guarantees.
Specifications, certification, design, and schedule are confirmed to de-risk scope and budget.
BOM and circuit feasibility are reviewed so performance and compliance standards are met.
Functional and image tests, including DICOM-compliant customized displays, validate results early.
EMC/ESD, protection, and durability tests are performed to secure stability in clinical use.
IEC 60601-1 and CE MDR documentation are supported to accelerate market entry.
Trial runs and tooling are tuned to ensure readiness for scale.
Strict QC ensures reliable supply and lifecycle management for customized medical displays.
Firmware, calibration, and accessory upgrades keep customized 4K UHD medical monitors accurate and reliable over time.
Reshin is a manufacturer focused on OEM and customized manufacturing programs.
Customization is accepted only when it can be verified, documented, and repeated in production.
Yes, when key configurations are frozen after validation and managed under change control.
Yes. Pilot runs are recommended to confirm repeatability and integration stability.
Key parameters are frozen, and changes follow a documented control process.
We provide technical documentation and engineering cooperation for customer-led submissions.
For selected projects, SKD is supported when logistics and regulatory conditions are feasible.
Application, target configuration, interfaces, mechanical constraints, target market, volume range, and timeline.
To provide the best experiences, we use technologies like cookies to store and/or access device information, Consenting to thesetechnologies will allow us to process data such as browsing behavior or unique lDs on this site, Not consenting or withdrawing consentmay adversely affect certain features and functions.
We will contact you within 1 working day, please pay attention to the email with the suffix “@reshinmonitors.com”.
Fill in the key details and we’ll reply with a solution soon.please pay attention to the email with the suffix “@reshinmonitors.com”.
We will contact you within 1 working day, please pay attention to the email with the suffix “@reshinmonitors.com”.