European healthcare procurement requires 4K medical displays to navigate complex regulatory frameworks where technical capabilities must align with systematic compliance validation, clinical safety requirements, and ongoing documentation obligations that vary based on intended medical applications.
4K medical-grade displays for European markets must meet MDR compliance requirements, demonstrate safety and EMC performance in clinical environments, deliver stable clinical performance across diverse workflows, and maintain comprehensive documentation supporting audit-ready verification and post-market surveillance obligations.

In my experience supporting European medical display deployments at Reshin, moving to 4K has changed the evaluation criteria from “spec sheet performance” to “system behavior under clinical constraints.” European tenders increasingly expect a clear, auditable framework: intended use drives the regulatory pathway, system-level safety and EMC must be proven in real environments, and clinical performance must be verified as repeatable and maintainable across rooms and sites.
Unlike markets where peak performance can dominate the decision, European deployments tend to reward evidence that 4K capability1 improves workflows without creating new failure modes, compliance gaps, or maintenance risks. That pushes engineering teams to treat compliance as a sustained delivery system—commissioning baselines, change control, and ongoing documentation continuity—not a one-time launch activity.
Are you regulated as an EU MDR medical device or accessory?
Determining MDR regulatory status requires systematic analysis of intended clinical use, manufacturer claims, and the display’s role in medical decision-making processes, with classification directly affecting compliance obligations and documentation requirements.
MDR classification depends on intended use statements and clinical purpose claims. Displays intended for diagnostic interpretation, surgical guidance, or patient monitoring typically qualify as medical devices requiring CE marking, while those used for general information display may avoid MDR requirements through careful intended use definition.
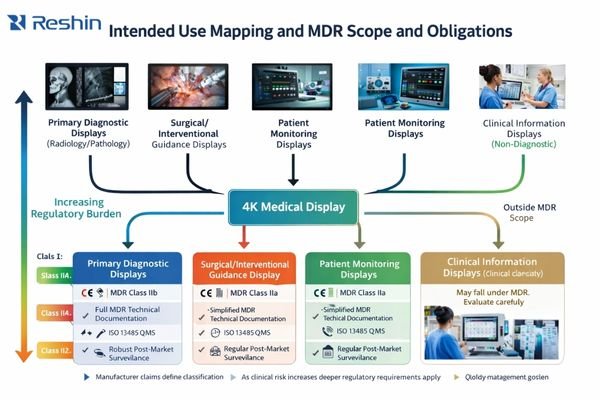
Define an auditable intended use baseline
From my work on European regulatory submissions, the first critical step involves establishing a defensible, auditable intended use definition that clearly positions the display’s clinical role. This determination affects every subsequent compliance activity, from technical documentation requirements through post-market surveillance obligations. For 4K medical displays, the regulatory pathway depends not on display resolution but on how the device functions within clinical workflows and decision-making processes.
The intended use statement must clearly define whether the display serves diagnostic reading applications in radiology or pathology, provides intraoperative visualization for endoscopic or surgical procedures, or functions as a general information display for clinical review and communication. Once positioned as a clinical decision-support endpoint, compliance priorities shift to demonstrating basic safety and essential performance under reasonably foreseeable use and fault conditions.
Control essential performance risks and responsibility boundaries
Critical performance risks that must be addressed include incorrect geometry or aspect ratio that could affect clinical interpretation, color or brightness drift that might compromise clinical judgment, signal interruptions during input switching that could disrupt workflows, unpredictable lock times that affect operational efficiency, and abnormal latency that could interfere with surgical control systems. For integrators, responsibility boundaries must be clearly established early in the project: determining who serves as the regulatory manufacturer, who maintains technical documentation and risk management files, and who handles post-market surveillance and change control obligations.
Which EU safety and EMC expectations must your 4K display satisfy?
European clinical environments demand robust safety and electromagnetic compatibility performance that addresses the unique challenges of 4K signal processing in complex medical settings with multiple interference sources and critical safety requirements.
Safety and EMC compliance requires systematic validation of shock protection, leakage current control, fault condition behavior, and electromagnetic immunity performance. 4K displays face heightened EMC challenges due to higher bandwidth requirements and increased susceptibility to interference from surgical equipment and wireless devices.
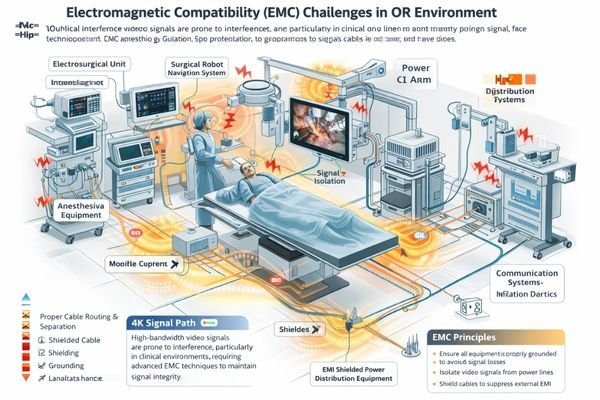
Treat safety and EMC as system behavior in real rooms
In EU hospital environments, safety and EMC validation extends beyond certificate compliance to encompass systematic verification of the display as part of integrated clinical systems. Operating rooms present particularly challenging environments where electrosurgical units, anesthesia equipment, navigation systems, wireless devices, and multiple power domains create complex electromagnetic interference patterns that can affect 4K signal integrity and display stability.
Engineer interference resilience for high-bandwidth 4K signal paths
4K displays with high-bandwidth signal processing face increased susceptibility to electromagnetic interference, making robust shielding, proper grounding strategies, and careful cable routing critical for maintaining signal integrity. Weak shielding, inadequate grounding, improper cable management, or connector strain can amplify interference effects, resulting in visible artifacts including signal flicker, frame drops, brief signal interruptions, re-lock events, or abnormal color behavior that could affect clinical interpretation.
From a system integration perspective, displays must function as reliable nodes within complex signal distribution architectures, demonstrating predictable behavior when connected through matrices, converters, extenders, fiber links, and isolation components. Recovery behavior must remain consistent under worst-case electromagnetic conditions, ensuring that clinical workflows aren’t disrupted by unpredictable display behavior during critical procedures. For comprehensive system-level EMC validation tailored to your specific clinical environment requirements, contact info@reshinmonitors.com to review an OR-specific EMC risk map2 and build an acceptance test plan that reflects your actual room environment.
What image quality and clinical performance must 4K deliver in practice?
European clinical deployments prioritize stable, repeatable 4K performance that enhances workflow reliability rather than simply providing increased resolution, with acceptance criteria focused on consistency and predictability across diverse clinical applications.
Clinical 4K performance requires low latency with stable geometry, consistent color reproduction across viewing conditions, predictable switching behavior, and maintained image quality under challenging environmental conditions. Performance validation must address workflow-specific requirements rather than generic display specifications.
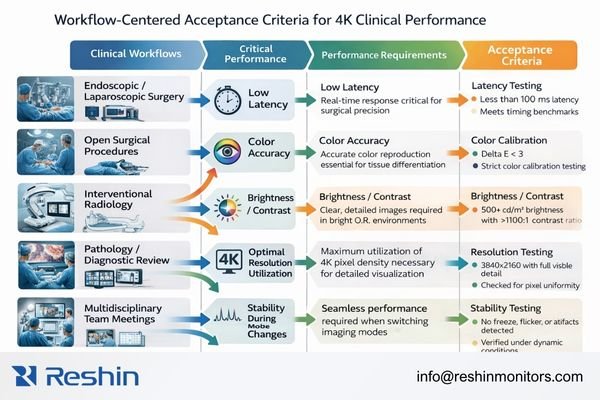
Translate “image quality” into commissioning acceptance criteria
Based on my analysis of European procurement requirements, clinical teams typically care less about peak 4K specifications and more about whether that resolution translates into stable, measurable clinical value across real workflows. The engineering move is to translate “image quality3” into acceptance criteria that can be validated during commissioning and re-validated after changes, rather than relying on subjective impressions.
In operating room and endoscopic workflows, the priority set usually includes minimal latency for real-time control, stable geometry without unintended scaling or aspect ratio changes, readability under bright ambient lighting with controlled reflections, and predictable lock/recovery behavior during source switching, menu overlays, and recording transitions. The display should behave consistently across input modes and processing states, without hidden changes that alter timing or framing during critical moments.
Maintain stable baselines across diagnostic, review, and multi-room deployments
In diagnostic reading and clinical review workflows, the emphasis shifts toward stable luminance behavior, consistent tonal response, surface uniformity, controlled long-term drift, and traceable QA routines that support institutional oversight. Digital pathology and multidisciplinary conference workflows add another layer: consistent color appearance, stable white point, and unified visual baselines across endpoints to support collaboration without “translation overhead” between rooms.
For procurement and integration, the most useful acceptance language is explicit: identical inputs should produce consistent outputs without silent color space shifts or bit depth reductions across ports, switching recovery should be repeatable and recorded, and high-bandwidth 4K modes should remain stable at maximum intended cable lengths under realistic interference conditions. In multi-room rollouts, the same approved modes and the same acceptance cases should be used room-to-room so consistency becomes a managed baseline rather than an assumption.
How to prove EU compliance with documentation, labeling, and verification?
European compliance validation requires comprehensive documentation that creates auditable traceability from regulatory requirements through design decisions, risk management, verification evidence, and ongoing post-market surveillance activities.
Compliance proof requires systematic technical documentation including risk management files, standards compliance evidence, verification test reports, proper labeling and instructions for use, and change control procedures that maintain regulatory integrity throughout the product lifecycle and clinical deployment phases.
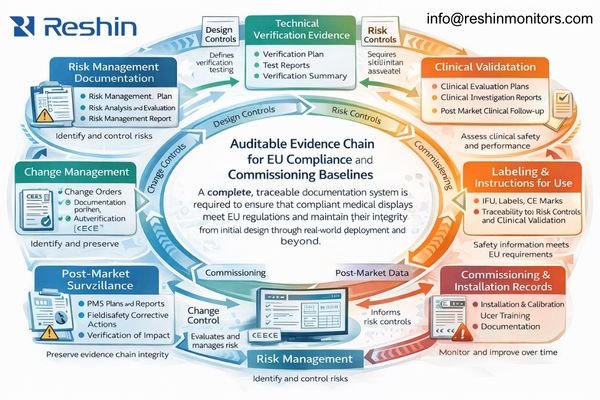
Build an auditable technical file aligned to claims and IFU
The documentation challenge involves building an auditable evidence chain4 that connects R&D, manufacturing controls, integration procedures, and operational maintenance into a coherent compliance narrative that satisfies both regulatory scrutiny and clinical expectations. In practice, documentation fails most often when claims, labeling, and real-world deployment behavior drift apart—so the goal is to keep intended use, IFU/labeling language, risk controls, and verification results aligned over time.
Technical documentation must demonstrate systematic risk management implementation that identifies potential hazards, evaluates clinical risks, implements appropriate control measures, and monitors residual risks throughout the product lifecycle. For 4K displays, this includes addressing risks associated with signal processing complexity, electromagnetic compatibility challenges, thermal management requirements, and potential failure modes that could affect clinical safety or workflow continuity.
Verify real deployment conditions and freeze a room baseline for change control
| Documentation Component | Required Evidence | Validation Methods | Maintenance Requirements |
|---|---|---|---|
| Risk management files | Hazard analysis, control measures, residual risk rationale | Control verification and traceability review | Updated with changes |
| Standards compliance | Safety/EMC/usability evidence packages | Accredited or witnessed verification where applicable | Reviewed as standards evolve |
| Clinical performance | Workflow validation and performance rationale | Commissioning evidence + field feedback loops | Ongoing monitoring |
| Manufacturing controls | Quality controls and process evidence | Audit trails and batch records | Continuous |
| Change control | Impact analysis and regression evidence | Re-verification against baseline cases | Applied to all modifications |
Verification evidence must include comprehensive test reports demonstrating conformity with applicable safety standards, electromagnetic compatibility requirements, and clinical performance specifications. Testing must address real-world deployment scenarios including worst-case use conditions, power sequencing scenarios, repeated switching operations, operational mode transitions, power loss recovery, maximum cable length performance, high-interference environments, and integration with various signal distribution equipment.
Commissioning activities become part of the regulatory evidence chain, requiring validation of complete systems under actual clinical conditions. This includes documenting acceptance test procedures that verify compliance-critical performance characteristics, installation qualification confirming proper environmental and safety conditions, operational qualification validating clinical workflow integration, and performance qualification demonstrating ongoing conformity with essential performance requirements.
Which Reshin 4K medical displays suit EU procurement and integration?
European deployment success requires selecting 4K displays based on clinical role requirements, integration complexity management, and fleet standardization capabilities rather than maximum technical specifications alone.
For EU projects, I recommend focusing on a small, standardizable set of displays that can hold a repeatable baseline across rooms and remain predictable after maintenance. Reshin’s 4K surgical display portfolio is selected in practice by how it behaves in the room: stable input handling, repeatable lock/recovery, controllable modes for baseline standardization, and integration characteristics that remain serviceable across multi-room deployments.
Standardize around a small model set for repeatable EU rollouts
| Clinical application | Integration requirements | Recommended model | EU deployment focus | Fleet management focus |
|---|---|---|---|---|
| Primary OR viewing | Stable input handling, EMC resilience | MS430PC | Repeatable OR behavior under interference | Standardized acceptance cases |
| High-performance surgical / teaching | 4K workflow support, predictable recovery | MS550P | Stable performance across mode changes | Unified room baselines |
| Multi-source integration | Converter/matrix compatibility, switching stability | MS322PB | Controlled negotiation boundaries | Repeatable commissioning |
| Flexible clinical deployment | Consistent behavior across rooms | MS321PB | Baseline standardization support | Simplified re-verification |
Map clinical roles to predictable integration behavior
For primary OR viewing positions and endoscopic visualization, the priorities are stable input behavior, predictable recovery during operational mode changes, and reliable performance in demanding electromagnetic environments—where MS430PC and MS550P are often used to anchor primary and teaching/overview positions. In rooms with complex routing through matrices, extenders, converters, or KVM, compatibility risk tends to come from negotiation uncertainty and conversion boundaries, so MS322PB and MS321PB are positioned as stable endpoints to help keep switching behavior and viewing baselines controlled and repeatable across changes.
FAQ
Is every "medical-grade" 4K display automatically an MDR medical device in Europe?
No, MDR classification depends on intended use and manufacturer claims about clinical purpose. Displays marketed for medical diagnosis, patient monitoring, or surgical guidance typically qualify as medical devices, while those positioned for general clinical information display may avoid MDR requirements through careful intended use definition and marketing claims.
Which documents and test evidence do EU tenders typically require beyond specs?
EU procurement commonly requires CE marking evidence, risk management documentation, safety and EMC test reports, clinical performance rationale, technical file summaries, and defined procedures for change control and post-market surveillance. Many tenders also request commissioning records and maintenance documentation requirements.
Do surgical 4K displays need the same calibration discipline as diagnostic displays?
Surgical displays often require calibration disciplines focused on stability, consistency, and geometry rather than the same grayscale-centric approach used for diagnostic reading. However, repeatable modes, routine verification, and traceable QA procedures are still important to maintain consistent clinical performance.
How can integrators prove stability across DP/HDMI/SDI and converter chains?
System stability requires end-to-end validation: maximum cable length tests, repeated switching behavior across operational modes, interference-aware stress tests, and documented recovery procedures. The most useful evidence is a room baseline that can be re-run after any change.
What environmental and recycling obligations should EU purchasers verify?
Purchasers should verify supplier declarations and documentation for environmental and recycling obligations relevant to electrical/electronic equipment, including substance declarations, take-back/recycling arrangements where applicable, and required markings and procurement documentation.
Conclusion
EU requirements for 4K medical-grade displays form a practical framework that ties regulatory scope, system-level safety and EMC behavior, workflow-based performance verification, and audit-ready documentation into one deployable approach. The most reliable projects convert “requirements” into repeatable room baselines: defined viewing modes, controlled input and conversion boundaries, stress-tested switching and recovery behavior, and QA records that can be re-verified after changes.
As a Reshin engineer, I see the best EU deployments succeed when compliance and integration are treated as one engineering system—intended use, risk controls, verification evidence, commissioning baselines, and change control all aligned—so 4K capability remains stable and auditable across the fleet. Contact the Reshin team at info@reshinmonitors.com to review your EU deployment scope and build an acceptance baseline that stays repeatable through upgrades and maintenance.
-
Explore how 4K capability enhances clinical workflows and safety in medical environments. ↩
-
Exploring EMC risk maps can provide insights into maintaining safety and compliance in complex hospital environments. ↩
-
Understanding how to define and measure image quality can enhance clinical workflows and improve patient outcomes. ↩
-
Understanding the concept of an auditable evidence chain is crucial for ensuring compliance and regulatory success in documentation. ↩



