Medical equipment buyers face overwhelming choices in medical-grade display technology. Which factors truly matter for clinical outcomes and long-term value in today’s complex healthcare environment?
As medical visualization continues to evolve, the importance of specialized medical-grade displays in ensuring diagnostic precision and surgical safety has never been greater, requiring procurement teams to adopt systematic evaluation approaches for both products and manufacturers at MEDICA 2025 Düsseldorf.

Drawing from my years of experience in medical display engineering, I’d like to share several practical insights into how procurement teams can evaluate display quality, verify manufacturer reliability, and prepare for global exhibitions such as MEDICA 2025 Düsseldorf, one of the most influential medical device showcases worldwide.
Five Key Factors to Consider When Purchasing Medical-Grade Displays (Buying Guide)
Healthcare facilities making incorrect display procurement decisions face potential diagnostic errors, workflow disruptions, and premature replacement costs. What essential evaluation criteria should guide your selection process?
Selecting reliable medical-grade displays requires looking beyond marketing specifications to focus on five critical areas: DICOM calibration precision, regulatory compliance documentation, environmental durability, interface flexibility, and comprehensive lifecycle support services.
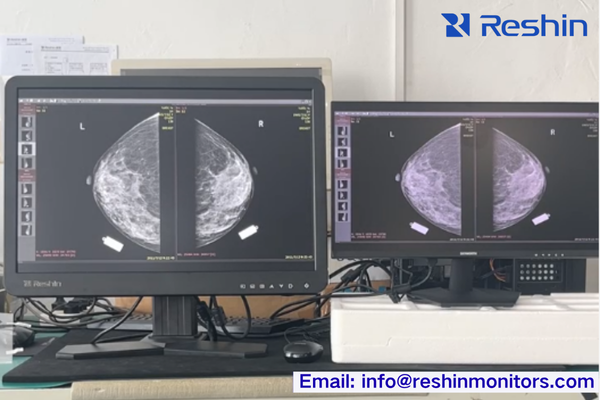
DICOM Calibration & Imaging Accuracy
Based on my experience calibrating hundreds of diagnostic displays, I can confirm that imaging accuracy fundamentally determines clinical utility. DICOM Part 14 compliance1 ensures consistent grayscale reproduction across different display models and manufacturers—essential for reliable diagnosis across networked hospital environments. When evaluating displays, request actual DICOM conformance test reports rather than relying on marketing claims.
I usually check for TG18 grayscale consistency, ΔL/L limits, and illumination conditions in the report instead of simply trusting “DICOM compliant” labels.
Regulatory Compliance & Certifications
Certification documentation provides objective evidence of manufacturing quality and regulatory compliance. Look specifically for CE MDR certification (not just CE marking), which confirms adherence to Europe’s rigorous Medical Device Regulation. Additionally, verify the manufacturer’s ISO 13485 certification, which demonstrates commitment to quality management systems specific to medical devices.
I always check for the Notified Body number and certificate validity period to avoid confusion with general CE markings.
Environmental Durability & Hygiene Design
Reliability and hygiene design features are especially critical in surgical environments. I recommend prioritizing displays with:
| Feature | Clinical Benefit | Verification Method |
|---|---|---|
| IP65/IP66 Front Protection | Prevents fluid ingress during procedures | Request test certification |
| Optical Bonding | Eliminates internal condensation; enhances contrast | Compare viewing angles in person |
| Anti-Microbial Housing | Reduces infection risk in sterile environments | Ask for independent lab results |
| Fanless Design | Minimizes airborne contamination | Verify heat dissipation method |
| Anti-Reflection Coating | Reduces eye strain during extended use | Check reflection percentage |
Interface Integration & Connectivity
Interface integration capabilities determine long-term utility as hospital systems evolve. Modern medical displays should support multiple input standards including 12G-SDI (for OR camera systems)2, DisplayPort 1.4, and HDMI 2.0.
For diagnostic workstations, I always verify 10-bit color signal chain integrity and PACS calibration software compatibility.
Lifecycle Support & Warranty Services
Lifecycle support significantly impacts total ownership cost. Evaluate warranty terms (industry standard is 3–5 years), calibration service availability, spare part commitments, and end-of-life policies.
I recommend requiring RMA service-level agreements and spare part availability periods in the purchase contract.
I’ve observed facilities achieve 7+ years of reliable service from properly supported displays, substantially improving return on investment.
Ready to evaluate medical displays for your facility? Contact our engineering team at info@reshinmonitors.com for a comprehensive assessment guide tailored to your specific clinical requirements.
How to Verify a Medical-Grade Display Manufacturer’s Real Capability
Medical technology buyers often struggle to distinguish between genuine manufacturers and assemblers of imported components. What evaluation methods reveal a supplier’s true production and engineering capability?
Reliable medical display manufacturing requires verifiable production systems, consistent quality metrics, authentic regulatory certifications, and responsive engineering support—four key areas procurement teams should investigate before partnership commitments.
Watch the video:
Factory tour (YouTube)
Production Environment Assessment
When visiting potential manufacturing partners, I first evaluate their production environment. True medical-grade capability requires ESD-protected assembly lines, darkroom calibration labs, aging test stations, and traceability barcode systems.
Request documentation of cleanroom classifications for optical assembly areas and verification of calibration equipment traceability.
Quality Metrics & Operational Performance
Quality and delivery records reveal operational stability. Request specific metrics including:
| Performance Indicator | Target Range (Typical) | Why It Matters |
|---|---|---|
| First-Pass Yield Rate | >95% | Indicates manufacturing process control |
| On-Time Delivery Performance | >98% | Predicts supply chain reliability |
| Product Return Ratio | <0.5% | Reflects quality control effectiveness |
| Mean Time Between Failures | >50,000 hours | Demonstrates long-term reliability |
| RMA Resolution Time | <14 days | Shows after-sales service capability |
| Engineering Change Response | <30 days | Indicates flexibility and adaptability |
Note: These target ranges are based on my past project experience and serve as a reference for procurement assessment.
These operational indicators predict whether a manufacturer can consistently support multi-year healthcare projects without supply disruptions.
Certification Authentication Process
Certification authentication is essential in an industry where documentation misrepresentation occurs. I recommend requesting notarized copies of:
- ISO 13485 certificates with scope covering display manufacturing
- CE MDR certification with the notified body number visible
- FDA registration documentation if US market access is required
- DICOM Part 14 compliance test reports from accredited laboratories
- (If applicable) HIPAA readiness for US data environments and UDI registration experience in Europe
Beyond verification, assess the issue and expiration dates3 of these certifications—recently obtained approvals may indicate limited experience with regulatory processes.
Engineering Support Capability
Engineering support capability differentiates value-adding partners from commodity suppliers. During evaluation meetings, discuss specific technical challenges to gauge expertise:
- Request examples of custom firmware development for specific clinical applications
- Ask about hardware modification capabilities for specialized environments
- Evaluate response times for technical support inquiries
- Determine if engineering documentation is available in multiple languages
- Confirm firmware update cycles and release consistency
I recommend preparing a structured capability assessment scorecard covering these four dimensions before engaging potential suppliers. This approach has helped me identify partners capable of supporting complex, multi-year medical imaging projects.
What to Look for When Attending a Medical Device Exhibition
Healthcare technology professionals often feel overwhelmed by the scale and complexity of major exhibitions. How can visitors efficiently identify valuable display partners at events like MEDICA 2025 Düsseldorf?
International exhibitions like MEDICA 2025 Düsseldorf offer opportunities to evaluate multiple display providers simultaneously, focusing on booth presentation quality, direct engineering access, live demonstration standards, documentation completeness, and appointment scheduling efficiency.
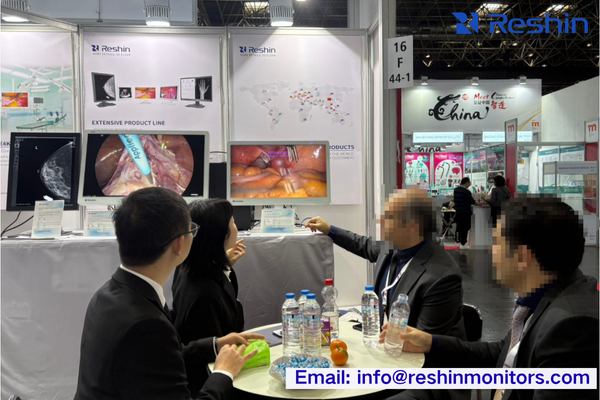
Booth Positioning & Professional Presentation
Having attended MEDICA for over a decade, I’ve developed a systematic approach to identifying valuable technology partners.
First, evaluate booth positioning and presentation quality4—established manufacturers typically secure prime locations in thematic pavilions and invest in professional demonstrations that accurately simulate clinical environments.
Technical Expertise & Engineering Access
Direct access to engineering expertise reveals real technical depth.
Instead of limiting interactions to sales representatives, credible manufacturers bring product developers and technical specialists to exhibitions. I recommend preparing questions in advance, such as:
- “How do you ensure DICOM calibration stability over the display lifecycle?”
- “What measures protect against signal interference in OR environments?”
- “How does your optical bonding process prevent delamination in high-humidity settings?”
The quality of answers reveals whether you’re speaking to true engineers or scripted presenters.
Live Demonstration Quality
Live demonstrations provide essential performance validation.
For diagnostic displays, observe grayscale transitions, viewing angle stability, and calibration interfaces.
For surgical monitors, test brightness uniformity, color accuracy under surgical lighting, and response to simulated fluid exposure.
I also confirm 12G-SDI transmission integrity and EMI resistance in OR setups.
Exhibition Preparation Checklist
| Timeline | Action Item | Purpose |
|---|---|---|
| 4 Weeks Before | Research exhibitor list and booth map | Strategic visit planning |
| 3 Weeks Before | Schedule meetings with key manufacturers | Ensure access to specialists |
| 2 Weeks Before | Prepare printed compliance checklist and questions | Enable structured evaluation |
| 1 Week Before | Download floor plan and map routes | Optimize time management |
| During Exhibition | Take notes and photos (with permission) | Record findings responsibly |
| Post-Exhibition | Request follow-up documentation | Validate technical claims |
Planning your MEDICA 2025 visit? Email info@reshinmonitors.com to pre-schedule a personalized demonstration of our medical-grade display solutions with our engineering team at Hall 16 D67-2.
Trending Types of Medical-Grade Displays in 2025
Healthcare facilities planning technology upgrades face difficult decisions about which display technologies will deliver sustainable clinical value. Which innovations are transforming medical visualization in 2025?
Medical display technology in 2025 converges toward higher resolution diagnostics, 4K surgical visualization, optically bonded designs for environmental protection, all-in-one clinical workstations, and antimicrobial materials for infection control.

Through my work developing calibration systems for medical displays, I’ve observed five major trends shaping clinical visualization in 2025:
12MP Diagnostic Displays
12MP (4K × 3K) diagnostic displays now enable multi-image comparison for mammography, CT, and MRI. Verify these features:
- Multi-modality layout management
- AI-assisted reading integration
- Sub-region luminance calibration
- Uniform brightness across surface
A 31-inch 12MP diagnostic model with AI calibration demonstrates the clinical advantages of this display class.
4K Surgical Monitors
4K surgical monitors are now standard for minimally invasive procedures. They feature:
- Wide color gamut (>110% sRGB)
- High brightness (700+ cd/m²)
- Ultra-low latency (<15ms)
- Multi-source display in OR systems
I prioritize verifying 12G-SDI long-distance signal quality and EMI interference protection in surgical suites.
Optically Bonded Panels
Optical bonding eliminates the air gap between the protective glass and LCD panel.
I test displays in thermal shock and high-humidity environments to check for fogging or delamination.
Key benefits include improved contrast, condensation resistance, and impact durability.
All-in-One Medical Workstations
All-in-one workstations integrate computing and calibrated displays. I always confirm:
- DICOM calibration
- Antimicrobial housing
- Infection-control sealing
- Cable and thermal management
- Software and domain compatibility for hospital IT policies
Antimicrobial Design Technologies
Modern infection control standards drive adoption of antimicrobial materials:
silver-ion housings, seamless edges, and chemical-resistant coatings that withstand hospital disinfectants.
| Display Technology Trend | Key Specifications | Clinical Benefits | Target Applications |
|---|---|---|---|
| 12MP Diagnostic Displays | 4200×2800, 1000+ cd/m², AI calibration | Enhanced diagnostic accuracy | Radiology, Mammography |
| 4K Surgical Monitors | 3840×2160, 700+ cd/m², IP65 protection | Superior tissue visualization | Surgery, Endoscopy |
| Optically Bonded Panels | Zero air gap, wide 170° view, reflection reduction | Clearer images, condensation resistance | ORs, Mobile Carts |
| All-in-One Workstations | Integrated computing, antimicrobial housing | Space optimization, maintenance ease | PACS, Clinical Review |
| Antimicrobial Designs | Silver ion materials, seamless build | Reduced infection risk, longer lifespan | ICU, Patient Rooms |
These innovations support the industry’s evolution toward intelligent visualization and safer clinical environments.
When planning 2025–2030 upgrades, prioritize displays that integrate these advancements for maximum longevity.
Interested in exploring how these advanced display technologies can benefit your facility? Contact info@reshinmonitors.com for a detailed technology roadmap assessment.
Visit the Booth of China’s Largest Medical-Grade Monitor Manufacturer at MEDICA 2025 Düsseldorf
Healthcare technology professionals have limited time at major exhibitions. How can you ensure productive engagement with display specialists during your MEDICA visit?
Connect with our engineering team at Hall 16 D67-2 during MEDICA 2025 Düsseldorf, where we’ll showcase our complete medical-grade display demos from November 17–20.
📍 Venue: Düsseldorf Exhibition Centre, Stockumer Kirchstraße 61, D-40474 Düsseldorf, Germany
📅 Date: November 17–20, 2025
🕒 Opening Hours: 10:00–18:00 (Nov 17–19); 10:00–16:00 (Nov 20)
🏢 Booth: Hall 16 D67-2
As calibration engineer, I’ll be personally conducting demonstrations throughout the exhibition.
Our booth is located in the Imaging & Diagnostic Equipment zone, with easy access to related technology providers.
Visitors can schedule private sessions by emailing info@reshinmonitors.com to arrange discussions on integration, quality testing, or OEM cooperation.
Our live MEDICA showcase will feature:
- DICOM calibration verification
- Optical bonding & splash-resistance tests
- Signal processing for surgical visualization
- Integration with major PACS and OR systems
- Customization options for OEM partners
Whether you’re a hospital procurement specialist, integrator, or medical device developer, our engineering team will be available for focused consultations on display innovation and workflow optimization.
Conclusion – Advancing Medical Display Excellence Together
The trend at MEDICA 2025 Düsseldorf is clear: medical technology is becoming smarter, more connected, and more patient-centered.
High-performance medical-grade displays remain the visual foundation of this transformation, empowering clinicians to diagnose with confidence and operate with greater precision.
💡 Let’s continue this conversation — I’d be glad to discuss how next-generation medical display technologies can enhance your diagnostic accuracy, surgical efficiency, and overall workflow integration.
📩 Connect with our engineering team (info@reshinmonitors.com) to explore tailored medical display solutions built for your unique imaging and integration requirements.
Whether you’re looking for standard configurations or fully customized OEM/ODM solutions, our experts are ready to deliver reliable, certified systems designed for long-term performance and clinical excellence.
🤝 Reshin — your trusted partner in professional medical visualization solutions.
-
Understanding DICOM Part 14 compliance is crucial for ensuring imaging accuracy and reliable diagnoses in clinical settings. ↩
-
Explore this link to understand how 12G-SDI enhances video quality and integration in operating rooms. ↩
-
Understanding the significance of issue and expiration dates can help ensure compliance and reliability in certifications. ↩
-
Understanding booth positioning and presentation quality can enhance your event strategy and partner selection. ↩



