In my experience supporting healthcare procurement processes, I’ve seen how poorly structured RFPs lead to compliance gaps, performance drift, and lifecycle management problems that surface months after installation when system changes reveal inadequate acceptance criteria.
Medical-grade monitor RFPs should include enforceable clauses covering compliance traceability, measurable performance acceptance criteria, documented baseline configurations, and lifecycle controls that prevent drift after updates and replacements. Strong RFP language converts operational requirements into verifiable deliverables with defined test methods and pass/fail criteria.
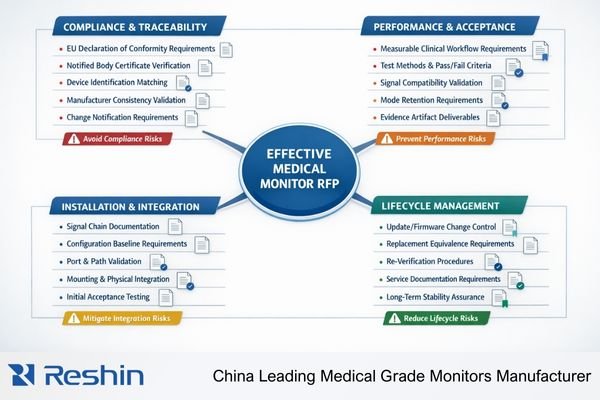
From my work with procurement teams, the most effective RFPs treat medical monitor acquisition1 as system assurance rather than hardware shopping, focusing on repeatability and traceability that supports clinical workflows throughout the entire service lifecycle. As a medical-grade monitor manufacturer, we see the difference between “it met the spec on day one” and “it stays consistent after updates, switching, and service”—and the RFP is where buyers can lock that consistency into enforceable deliverables.
Why Do RFP Clauses Matter More Than Spec Sheets for Medical-Grade Monitors?
RFP clauses address operational realities that specifications alone cannot capture or guarantee.
Spec sheets rarely capture operational realities driving clinical risk and lifecycle cost: repeatability after updates, signal-chain behavior during switching, cleaning constraints, and compliance traceability across replacements. Strong RFP clauses convert hidden variables into enforceable acceptance criteria defining what must be delivered, verification methods, and consequences for performance drift.
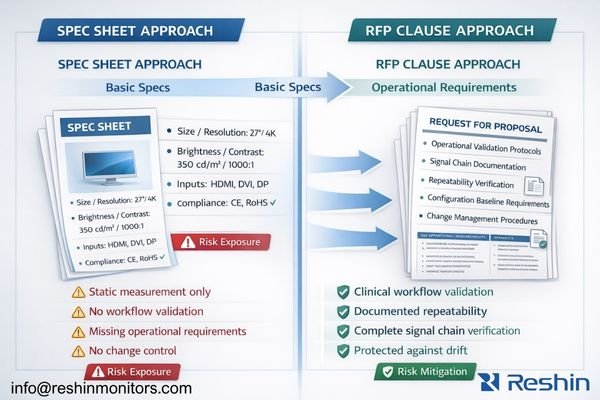
In my OR integration work at Reshin, I’ve learned that RFPs prevent silent downgrades, scope mismatches, and documentation gaps that typically surface during audits, incident reviews, or urgent downtime. The key shift is moving from “what the product can do in theory” to “what the deployed system must do repeatedly,” with evidence artifacts that survive staff turnover, routine maintenance, and equipment replacement.
Hidden Operational Variables
Medical monitor performance depends on more than published specs: the real risks come from signal-chain interactions2, mode negotiation behavior, and drift after routine events like power cycles, OS/GPU updates, or switching through intermediate devices. In clinical environments, “works most of the time” is not an acceptable state, because intermittent fallbacks and silent presentation changes create workflow friction and reduce confidence. Strong RFP language should name these variables explicitly so they can be tested and documented.
Enforceable Acceptance Standards
RFP clauses turn qualitative needs into measurable deliverables by defining acceptance tests, pass/fail outcomes, and required evidence packages. Instead of accepting “image is visible,” you can require repeatability cycles, baseline configuration files, and verification records that prove the system returns to the same validated mode after predictable transitions. This is also where buyers define accountability: what constitutes nonconformance, what remediation looks like, and how replacement units must match validated behavior rather than just “similar specifications.”
Which Compliance and Traceability Clauses Reduce Procurement Risk Fast?
Compliance clauses should create verifiable connections between regulatory documentation and delivered devices.
Effective RFPs make compliance verifiable and tied to delivered configurations: require signed EU Declaration of Conformity when applicable, scope evidence matching intended medical purpose, and labeling consistency linking legal manufacturers to exact model and revision delivered. Include notification and re-verification processes for changes affecting compliance or labeling to prevent silent product substitutions.
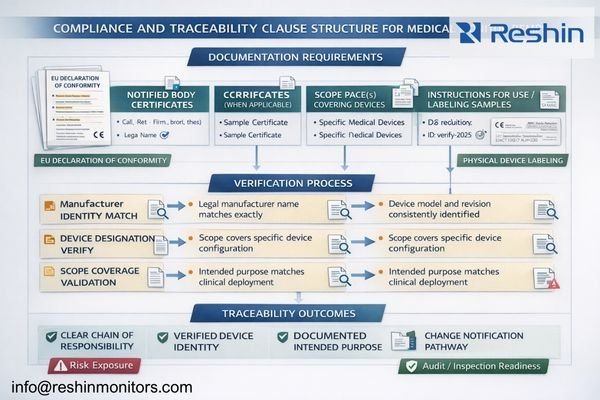
Based on the projects I support with PACS and KVM partners, the fastest risk reduction comes from closing the loop between paperwork and the physical unit: the same legal manufacturer identity3 and the same exact device designation must match across documents and labeling. Your RFP should state that submission packages must be traceable to the delivered configuration, not generic “certificates,” and that missing or inconsistent identifiers trigger a stop-and-escalate review before acceptance.
Include a clear bidder submittal checklist so evaluators can verify completeness quickly:
- Signed EU Declaration of Conformity (where applicable), naming the legal manufacturer and the exact device designation
- Certificate scope details (where applicable) that plausibly cover the intended medical purpose and the delivered configuration
- IFU/labeling samples showing intended purpose and manufacturer/responsible-party consistency
- Device identifier format (model/revision/serial, and any applicable UDI elements) and how it maps to documentation
- Change-notification commitments for firmware/hardware revisions and supply-chain substitutions affecting labeling or compliance evidence
Traceability clauses should also control lifecycle changes: define what constitutes a “reportable change,” require advance notification, and require re-verification when a change could affect labeling, compliance evidence, or the validated operating baseline. This prevents “equivalent replacement” from becoming an unintended substitution that breaks audit readiness or introduces operational differences after service events.
What Performance and Image-Quality Acceptance Criteria Should Be Written into the RFP?
Performance clauses should define measurable acceptance criteria tied to clinical workflows rather than generic specifications.
Instead of listing peak specifications, define measurable acceptance criteria tied to clinical workflows: required native input modes, predictable switching and re-lock behavior, and restrictions on unintended scaling or processing that could change perceived sharpness. RFPs should specify how acceptance will be tested with defined test patterns, repeatability cycles, and pass/fail thresholds.

Performance verification requires systematic testing that can be repeated after updates and replacements, not one-time “looks good” checks. The RFP should specify test patterns, transition cycles (boot/wake/switching), what constitutes the intended operating mode, and what evidence artifacts must be delivered at handover so the hospital can re-validate after change events.
| Clinical Application | Acceptance Criteria | Test Method | Pass/Fail Threshold | Documentation Required |
|---|---|---|---|---|
| Diagnostic Reading | Pixel-accurate presentation, calibration/QC readiness | Test patterns, calibration verification | Per site acceptance plan / Pending Confirmation | Calibration records, QC baselines |
| Surgical Viewing | Mode retention, switching/re-lock repeatability | Transition timing, mode verification | Per workflow acceptance plan / Pending Confirmation | Mode logs, switching records |
| Team Viewing | Uniformity and viewing-angle stability for shared observation | Multi-position checks, uniformity verification | Per site acceptance plan / Pending Confirmation | Uniformity records, verification notes |
| Multi-Input Systems4 | Cross-input consistency and negotiation stability | Cross-input repeatability cycles | No unintended mode drift across inputs | Input comparison records, repeatability logs |
| Long-Term Stability | Presentation stability during extended sessions | Extended operation observation | No clinically relevant drift vs baseline | Stability notes, baseline references |
For diagnostic applications, require deliverables that support ongoing QC programs (baseline settings, calibration records, and re-check procedures). For surgical viewing, emphasize predictable re-lock behavior and stable mode retention through real OR transitions such as power cycling, input switching, and routing changes. In all cases, enforceability comes from writing the test method and evidence artifacts into the RFP so acceptance is objective, repeatable, and auditable.
Which Integration, Installation, and Service Clauses Prevent Lifecycle Drift?
Lifecycle management clauses should prevent unmanaged changes that degrade clinical performance over time.
Lifecycle drift often results from unmanaged changes including OS updates, port modifications, new switchers, or replacement units with different behavior, so RFPs should require documented baseline configurations for complete signal chains and restoration methods after maintenance. Include installation deliverables and repeatability verification requirements after installation and major changes.
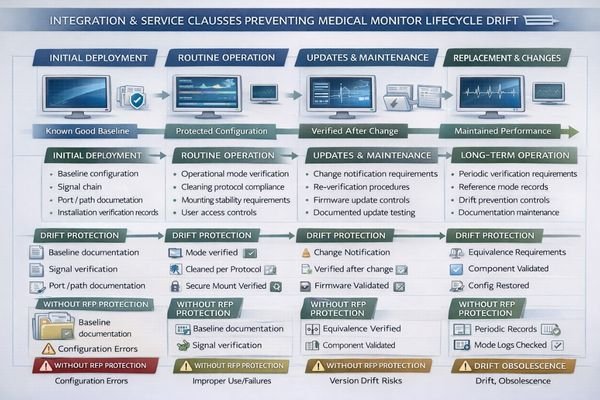
When I review systems that degrade over time, the pattern is usually simple: the original validated configuration was never captured in a recoverable way, and maintenance introduced untracked changes. RFP clauses should therefore require a baseline package for the complete signal chain (ports/paths, timing/encoding assumptions, and key device settings), plus a restore method after service, so the hospital can return the system to a known-good state instead of “rediscovering” it.
Installation and Configuration Control
Installation clauses should require documented port/path usage, cable and adapter rules, and configuration baselines for the workstation/GPU and monitor inputs used clinically. Include constraints that affect long-term stability—mounting, cable strain relief, and cleaning compatibility—because these often drive unplanned port swaps or intermittent connection behavior. Require acceptance verification across real transitions (power cycles, wake events, input switching) on the exact clinical path, with records delivered at handover so local teams can repeat the same checks after updates.
Service and Replacement Standards
Service clauses should define spares strategy and replacement equivalence tied to device identifiers (model/revision) and validated behavior, not just “similar size and resolution.” Require change notification for firmware/hardware revisions and mandate re-qualification after any change that could alter modes, scaling behavior, or compliance labeling. This keeps the deployed system stable across the service lifecycle5 and prevents “silent drift” that only appears when clinicians compare workstations or when an audit requires traceable evidence of what was deployed. Contact us at info@reshinmonitors.com if you need assistance developing comprehensive lifecycle management clauses for your medical monitor RFP requirements.
What Monitor Categories Fit Typical RFP Scenarios and How Should Buyers Specify Them?
Monitor specification should start with clinical scenarios and translate them into enforceable requirements.
Specification approaches should define clinical context and signal environment before translating requirements into measurable deliverables.
Start by specifying clinical scenarios and signal environments, then translate into enforceable requirements based on actual workflow needs rather than generic specifications.
| Clinical Role / Application | RFP Focus Areas | Key Requirements | Recommended Model | Specification Priorities |
|---|---|---|---|---|
| Diagnostic Reading | Grayscale accuracy, calibration | Consistent grayscale, pixel-accurate presentation | MD33G | Calibration stability, QC compatibility |
| Multi-Modal Clinical | Versatility, reliability | Multi-input consistency, stable operation | MD52G | Input stability, operational reliability |
| Large Format Diagnostic | Large display clinical | Large format with diagnostic accuracy | MD85CA | Large format precision, clinical compliance |
| Surgical Visualization | OR environment, switching | Fast re-lock, stable modes through transitions | MS321PB | Switching performance, OR environment validation |
| Premium Surgical Display | Advanced OR applications | Maximum reliability, premium performance | MS430PC | Premium reliability, advanced surgical features |
Diagnostic reading applications need consistent grayscale behavior with calibration readiness and pixel-accurate presentation, while surgical viewing prioritizes stable mode locking, fast switching, and predictable behavior through OR state transitions. Buyers should define size and resolution in terms of viewing distance and workflow (single-user reading versus shared team viewing), then lock down the signal assumptions: direct connection or routed chain, required inputs, and permitted adapters that are validated for the environment.
To keep the RFP enforceable over time, specify what must be documented at handover (port/path definitions, validated modes, baseline OS/GPU settings) and require a simple re-verification procedure after updates, maintenance, or replacements. This approach purchases repeatability: the same clinical path should return to the same validated presentation without unexpected scaling, silent fallbacks, or mode drift that complicates operations months after installation.
FAQ
Should an RFP ask for "certificates," or for specific MDR/CE evidence?
Ask for traceable evidence tied to the delivered configuration—such as a signed EU Declaration of Conformity and scope details (when applicable)—not generic certificates that don’t match the exact model/revision.
What’s the single most important acceptance clause for clinical consistency?
Repeatability across real transitions: the system must return to the same validated mode and presentation after power cycles, updates, and input switching.
How can buyers prevent "silent downgrades" after replacement or service?
Require replacement equivalence tied to model/revision identifiers plus a change-notification and re-verification process after any firmware/hardware changes.
Do buyers need different clauses for diagnostic vs surgical use?
Yes—diagnostic RFPs should emphasize calibration/QC and pixel-accurate behavior, while surgical RFPs should emphasize switching, re-lock speed, and stable modes through OR transitions.
What should be documented at installation handover?
Port/path usage, validated input modes, baseline OS/GPU settings, and a short verification checklist that can be repeated after updates or maintenance.
How do you keep RFP requirements enforceable rather than aspirational?
Write test methods and pass/fail criteria into the acceptance section, and require evidence artifacts (records, checklists, baseline files) as deliverables.
Conclusion
Strong medical-grade monitor RFPs transform clinical consistency requirements into enforceable procurement standards through traceable compliance evidence, measurable acceptance criteria, documented baseline configurations, and lifecycle controls that prevent performance drift after updates and replacements. When buyers define systematic test methods and repeatability expectations upfront, procurement evolves from specification shopping to system assurance that reduces audit risk and protects clinical workflows throughout the complete service lifecycle.
Our approach at Reshin emphasizes RFP development that addresses the operational realities of medical monitor deployment, including signal chain interactions, lifecycle management, and compliance traceability that supports both initial acceptance and long-term clinical operation. By incorporating enforceable acceptance criteria, systematic verification procedures, and comprehensive lifecycle management requirements, healthcare organizations can procure medical monitor systems that maintain validated performance characteristics across realistic operational conditions. The most effective RFP strategy treats medical monitor procurement as a system-level assurance process that extends throughout the equipment lifecycle, ensuring that clinical workflows remain supported by reliable, compliant, and consistently performing display systems that meet both immediate operational needs and long-term institutional requirements.
✉️ info@reshinmonitors.com
🌐 https://reshinmonitors.com/
-
Understanding best practices in medical monitor acquisition can enhance procurement strategies and ensure quality in clinical workflows. ↩
-
Understanding signal-chain interactions is crucial for ensuring reliable medical monitor performance in clinical settings. ↩
-
Understanding the significance of legal manufacturer identity can enhance compliance and traceability in medical device projects. ↩
-
Learning about multi-input systems can enhance understanding of cross-input consistency and operational stability. ↩
-
Exploring the service lifecycle helps in grasping how to manage and maintain systems effectively throughout their operational life. ↩