In my experience supporting medical monitor procurements, I’ve encountered numerous instances where CE markings and compliance claims don’t match the actual regulatory documentation, creating procurement risks for healthcare organizations.
Buyers can quickly verify CE/MDR compliance authenticity by checking document consistency across the EU Declaration of Conformity, applicable Notified Body certificates, and physical device labeling. The fastest verification focuses on matching legal manufacturer identity and specific device designations across all documentation, rather than relying on CE logos or marketing claims alone.

From my work with procurement teams, authentic compliance verification requires understanding the difference between legitimate regulatory documentation and superficial compliance claims that may not support actual medical device deployment. The key insight is that authentic compliance1 creates a traceable evidence chain connecting the delivered product to validated regulatory documentation, while questionable compliance often breaks down when subjected to systematic document review and physical verification procedures. A useful buyer rule is simple: if the legal manufacturer and exact device designation do not match across documents and the physical unit, treat it as a procurement red flag and escalate review.
What Does "Authentic CE/MDR Compliance" Mean for Medical-Grade Monitors?
Understanding authentic compliance helps distinguish legitimate regulatory submission from superficial marketing claims.
Authentic CE/MDR compliance means devices are legitimately placed on the EU market under the Medical Device Regulation with correct classification, valid EU Declaration of Conformity, proper labeling including appropriate CE marks and Notified Body numbers, and conformity assessment routes matching intended medical purposes—not just CE logos on bezels or generic certificates.
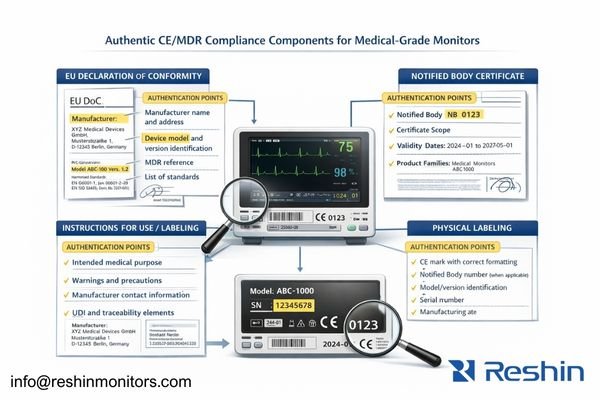
When I review compliance documentation for medical monitor installations, the practical goal is traceability: connecting the unit you received to a specific legal manufacturer, a specific device designation (model and version/revision), an intended purpose consistent with clinical deployment, and a conformity assessment scope that plausibly covers that exact product. Buyers should focus on what can be verified quickly and consistently, not on how polished a “certificate” looks.
Legal Manufacturer and Product Traceability
Authentic compliance ties the legal manufacturer (the entity responsible for the MDR declaration) to the exact device designation delivered to your site. The names and addresses should match across the EU Declaration of Conformity, any applicable certificate scope pages, and the physical label. The device identity on the label (model, revision/version, serial number, and any applicable UDI elements) should allow your team to prove that the delivered unit is the same product described in the documentation set, not just a similar-looking family member.
Conformity Assessment Route Validation
MDR compliance2 depends on the device’s intended purpose and risk classification, which determine the conformity assessment route and whether a Notified Body is involved. Buyers don’t need to become regulatory experts to spot risk: the documentation should clearly indicate MDR context, be tied to a specific product designation, and be internally consistent. If the supplier can’t explain how the documents relate to the delivered unit using traceable identifiers (rather than marketing claims), the procurement risk is high and warrants escalation to compliance or legal review.
Which Documents Can You Check in 10 Minutes to Confirm MDR Claims?
Rapid document verification focuses on consistency across core compliance documentation.
Start with three items that must align perfectly: the EU Declaration of Conformity showing manufacturer details, device designation, and applied standards; Notified Body certificate details covering scope and validity; and Instructions for Use/labeling showing intended purpose and manufacturer information. The fastest authenticity test is consistency across legal manufacturer identity and device designations.

In my OR integration work at Reshin, I’ve learned that consistency is the most reliable authenticity signal because legitimate compliance naturally produces aligned documents, while weak or recycled claims tend to show small mismatches. In 10 minutes, aim to confirm one closed loop: the same legal manufacturer name/address and the same exact device designation appear on the EU DoC, the certificate scope pages (if applicable), and the delivered unit’s label.
The EU Declaration of Conformity3 should read like a formal accountability document: legal manufacturer identity, exact device designation, MDR references, applied standards, and an authorized signature/date. If a Notified Body certificate is applicable for the device and route, the Notified Body identity and certificate scope should plausibly cover the device type and intended medical purpose, and the delivered model/version should be clearly within scope. The IFU and labeling should reinforce intended purpose, warnings, and responsible-party details in a way that matches the same manufacturer and the same product designation.
How Do You Validate the CE Mark, Labeling, and Traceability on the Physical Unit?
Physical device validation confirms documentation consistency with delivered products.
Verify unit labeling supports medical device traceability rather than consumer electronics styling: correct CE mark presentation, appropriate Notified Body numbers when required, legal manufacturer name and address, and consistent EU responsible-party information. Device identifiers on labels must match documentation, because authenticity often breaks when family certificates are presented for different product variants.

Physical validation is where procurement claims either connect to reality or fall apart. Your goal is not to judge aesthetics—it is to confirm the delivered unit can be uniquely tied to the same product described in the document set. Start with the label: confirm the legal manufacturer identity, then confirm the exact device designation (model and version/revision) and serial number match what you received in documentation and what you intend to deploy clinically. If the unit identifier cannot be matched cleanly, do not treat the claim as authenticated.
| Labeling Element | Verification Method | Authenticity Indicator | Common Problems | Risk Assessment |
|---|---|---|---|---|
| CE Mark Format | Visual presentation check | Correct size, format, placement | Incorrect proportions, wrong placement | Logo authenticity issues |
| Notified Body Number | Cross-reference with certificate | Matches certificate NB number | Missing when required, incorrect number | Assessment route problems |
| Legal Manufacturer4 | Compare with EU DoC | Exact name and address match | Brand vs legal entity mismatch | Responsibility traceability |
| Device Identifier | Match with documentation | Model, revision, serial alignment | Family cert for wrong variant | Scope coverage problems |
| UDI Elements | Traceability database check | Consistent UDI-DI assignment | Missing or incorrect UDI | Market surveillance issues |
Device identifier alignment is the most important checkpoint because it prevents a common failure mode: documentation that is valid for a related product family but not for the specific variant delivered to your facility. If procurement and clinical teams rely on “close enough” identifiers, problems later become hard to resolve during audits, incident reporting, or replacement cycles. Treat a clean label-to-document match as the minimum acceptance condition before moving on to technical evaluation.
How Can Buyers Cross-Check Notified Body Scope and Spot Common Red Flags?
Systematic scope verification provides efficient authenticity screening for procurement decisions.
Treat scope verification as a shortcut to authenticity: check whether listed Notified Bodies are legitimate and whether certificate scope covers the specific device type and intended medical purpose of monitors, not generic electrical equipment statements. Verify certificate scope pages list covered device families, confirm legal manufacturer identity matches contract entities, and ensure claimed standards align coherently across documents.
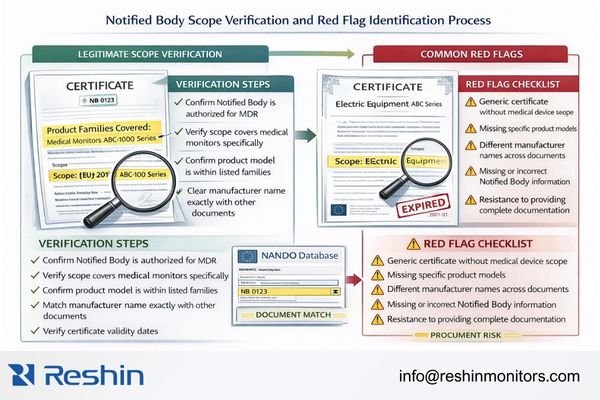
Scope verification is where many questionable claims fail quickly: authentic documentation usually includes scope language that makes sense for the device type and intended purpose, while weak claims hide behind generic categories. Buyers should request the scope pages that show what is actually covered, then confirm the delivered device designation is included without ambiguity and the legal manufacturer identity matches what appears on the EU DoC and the unit label. If any key identifier conflicts, pause procurement and escalate rather than trying to “interpret” the mismatch.
Certificate Scope Analysis
When a Notified Body certificate is applicable, the scope should read like a coverage statement for a medical device family, not a generic electronics certificate. Look for scope that plausibly maps to the monitor’s intended medical purpose and for device-family listings or codes that clearly include the delivered designation. The practical test is coherence: the scope language, the DoC device name, and the IFU intended purpose should not contradict each other. If the supplier cannot provide scope pages tied to your exact delivered configuration, treat the claim as unverified.
Red Flag Identification
Common red flags include “CE certificate5” documents without clear MDR context, certificates that do not clearly identify scope, mismatches between branding and legal manufacturer identity, reluctance to provide a signed EU DoC, and explanations that rely on marketing language instead of traceable identifiers. If any one of these appears, the fastest buyer-safe move is to stop technical comparisons and request a corrected, consistent evidence set tied to the exact device designation and label identifiers. Contact us at info@reshinmonitors.com if you need assistance developing systematic CE/MDR verification procedures for your medical monitor procurement processes.
How to Choose Medical-Grade Monitors with Lower CE/MDR Procurement Risk
Procurement risk reduction requires aligning compliance evidence with clinical deployment requirements before comparing specifications.
Risk reduction strategies should focus on evidence quality and vendor support capabilities rather than superficial compliance claims.
Define intended medical purpose clearly, whether for diagnostic review or surgical viewing, and confirm expected deployment environment including interfaces, switching behavior, and cleaning requirements before requesting compliance documentation packages.
| Clinical Role / Application | Compliance Focus | Documentation Requirements | Recommended Model | Risk Reduction Features |
|---|---|---|---|---|
| Diagnostic Reading | Medical device intended purpose clarity | DoC + labeling consistency for exact model/revision | MD26C | Traceable identifiers, consistent document set |
| High-Resolution Diagnosis | Scope coverage for specific variant | Scope pages covering delivered designation | MD52G | Clear designation coverage, repeatable labeling |
| Clinical Review | Coherent intended use language | IFU/label intended purpose matches DoC | MD22CA | Consistent intended purpose and labeling |
| Surgical Visualization | OR workflow readiness evidence | Document set tied to delivered configuration | MS270P | Repeatable configuration baseline support |
| Advanced Surgical Display | Lifecycle change control readiness | Documented change-management expectations | MS321PB | Support for baseline restore after service |
Lower procurement risk comes from purchasing a verifiable system state, not a logo. Start by aligning the intended purpose and deployment conditions with what you will actually do in the hospital—interfaces, switching behavior, mounting, cleaning, and service cycles—then require a documentation package that is traceable to the exact delivered configuration (model and revision/version identifiers) with consistent legal manufacturer identity across DoC, labeling, and any applicable scope evidence. Favor suppliers that can support lifecycle control: clear IFU/labeling, predictable configuration baselines, and change-management practices that preserve the same compliant configuration after firmware updates or replacement. As a medical-grade monitor manufacturer, Reshin focuses on traceable documentation, controlled configuration baselines, and lifecycle support processes that help procurement and clinical teams keep compliance and deployment behavior consistent over time.
FAQ
Is a CE logo on the monitor enough to prove MDR compliance?
No—buyers should confirm a traceable EU Declaration of Conformity, correct labeling, and (when applicable) a valid Notified Body scope that matches the device’s intended medical purpose.
What is the fastest authenticity check a hospital can do?
Consistency across documents and labeling: the legal manufacturer identity and the exact device designation must match on the EU DoC, certificate scope (if applicable), and the physical label.
Why do "certificates" sometimes look real but still be risky?
Because a document may be valid for a different product variant or scope; if the delivered model/revision is not clearly covered, the claim is operationally risky for procurement.
Does MDR always require a Notified Body number next to the CE mark?
Not in every scenario; it depends on classification and conformity assessment route, so buyers should verify what applies for the specific device and documents provided.
What should buyers request if the supplier refuses to share full details?
At minimum, a signed EU Declaration of Conformity and the certificate scope pages (when applicable) tied to the exact device designation, plus labeling/IFU evidence that matches the delivered unit.
How do intermediate devices or system integration relate to compliance verification?
Compliance is about the device and its intended use, but buyers should also ensure the delivered configuration and documented baselines support repeatable clinical operation in the actual signal chain.
Conclusion
Buyers can verify CE/MDR authenticity quickly by establishing a traceable, internally consistent evidence chain: the EU Declaration of Conformity, any applicable Notified Body scope, and the physical label must match the same legal manufacturer and the same exact device designation. This approach is faster and more reliable than relying on CE logos or marketing claims because authenticity typically fails at the points where identifiers, scope, and labeling should align.
At Reshin, we treat compliance verification as a procurement-quality process that must remain stable across the device lifecycle. By prioritizing scope and consistency, requiring documentation tied to the delivered configuration, and validating that identifiers match on paper and on the unit, healthcare organizations can reduce procurement risk while improving readiness for audits, service events, and long-term clinical deployment. The most effective strategy is repeatability: repeatable documentation, repeatable device identification, and repeatable support processes that keep the compliant state intact over time.
✉️ info@reshinmonitors.com
🌐 https://reshinmonitors.com/
-
Understanding authentic compliance is crucial for ensuring that medical devices meet regulatory standards and are safe for use. ↩
-
Exploring MDR compliance requirements helps ensure that your products meet necessary regulations and reduce procurement risks. ↩
-
Understanding the EU Declaration of Conformity is crucial for ensuring compliance and authenticity in medical devices. ↩
-
Verifying the Legal Manufacturer is crucial for accountability and traceability in medical device management. ↩
-
Exploring the significance of CE certificates can help buyers ensure they are making informed procurement decisions. ↩