Medical device manufacturers frequently waste hundreds of thousands of dollars on optical bonding rework, field failures, and supplier changes—all due to insufficient evaluation during initial selection. Which critical factors determine whether your optical-bonded monitor will be a competitive advantage or a costly liability?
Selecting an optical-bonded OEM medical monitor is not just about glass and glue; it’s about image performance, hygiene, reliability, and lifecycle risk. In my integration work with device manufacturers, I’ve found that the right partner and bonding process often matter more than the panel spec itself.
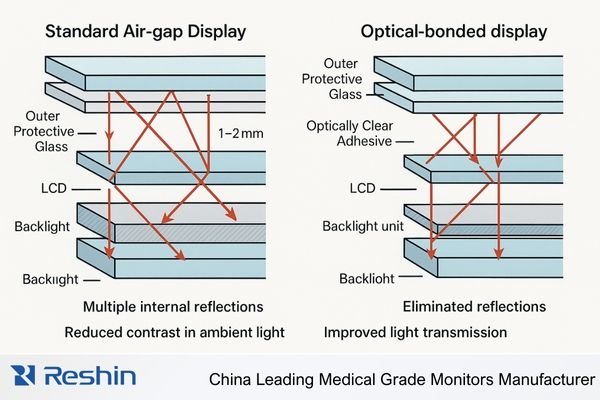
As medical devices become increasingly visualization-dependent, the performance of integrated displays directly impacts clinical outcomes, user satisfaction, and competitive differentiation. Optical bonding1—the process of permanently adhering protective glass to LCD panels using optically clear adhesives—represents a strategic investment that influences image quality, reliability, manufacturability, and lifecycle cost. For medical device product managers, R&D leaders, and supply-chain teams, the challenge is to move beyond spec sheets and evaluate optical-bonded OEM medical monitors in terms of clinical value, field reliability, and long-term risk.
What value does optical bonding really bring to medical OEM devices and monitors?
Medical device teams often hesitate to approve the premium for optical bonding because the benefits are hard to quantify on a spreadsheet and only show up later in real clinical use.
In my surgical and endoscopy integrations, optical bonding pays off when better contrast, hygiene, durability, and field reliability clearly outweigh its extra cost, process risk, and complexity across the full device lifecycle.

Optical bonding changes how the display behaves in clinical environments. Filling the air gap between LCD and cover glass cuts internal reflections, improves contrast in bright ORs, and stabilizes image quality across wider viewing angles. A seamless bonded front surface also improves cleanability, fluid resistance, and infection-control performance on carts, C-arms, endoscopy towers, and surgical robots. When you factor in fewer field failures, longer stable performance, and lower service and warranty costs, optical-bonded OEM medical monitors often deliver better total cost of ownership for devices used in bright, mobile, or fluid-exposed environments.
Clinical Value Factors
The primary justification for optical bonding in medical devices stems from its direct impact on clinical utility and performance:
-
Enhanced Image Clarity in Ambient Light
Traditional air-gap displays suffer from internal reflections that reduce contrast in brightly lit clinical environments. Optical bonding eliminates these reflections, maintaining image clarity under surgical lights, near windows, or in hybrid ORs. -
Improved Viewing Angle Performance
Air-gap displays often show contrast and color shifts at oblique angles. Optical bonding preserves image quality across wider viewing angles, supporting collaborative procedures where multiple clinicians must view the same display. -
Infection Control and Cleanability2
The seamless bonded surface eliminates crevices where contaminants can accumulate, creating a more cleanable surface that withstands frequent disinfection in high-risk surgical and interventional environments.
Operational Value Factors
Beyond clinical performance, optical bonding delivers operational benefits that impact total cost of ownership:
-
Enhanced Mechanical Durability
The bonded assembly increases impact resistance and robustness, reducing field failures in mobile applications like surgical carts and portable imaging systems. -
Moisture and Fluid Resistance
Superior edge sealing prevents liquid ingress from disinfectants, contrast media, and bodily fluids, reducing one of the most common failure modes for medical displays. -
Extended Lifecycle Performance
Properly bonded displays maintain optical performance longer by preventing internal contamination and reducing stress on LCD components, supporting longer service intervals.
Which optical performance metrics for optical-bonded medical monitors should device makers focus on first?
Device specifications often list dozens of display parameters, but only a handful of optical metrics truly determine whether the screen supports confident use in a real OR or lab.
When I help OEM teams specify optical-bonded medical monitors, I focus first on ambient contrast, reflection control, color accuracy, and uniformity under realistic clinical lighting—not only on lab numbers.
Instead of chasing every spec line, I encourage device manufacturers to prioritize a small set of clinically meaningful metrics. Ambient contrast ratio under defined lux levels shows how well structures remain visible in bright ORs. Specular and diffuse reflection levels reveal how effectively the optical bond and coatings control glare and haze. Matching color behavior to the camera or modality output, and keeping brightness and color uniform across the panel, ensures tissue differences and instruments appear consistently across the screen.
Environmental Contrast Performance
The most important optical characteristic for medical displays is their ability to maintain contrast under clinical lighting:
-
Ambient Contrast Ratio (ACR)
ACR quantifies contrast under specified ambient illumination. For surgical uses, targets of 30:1 at 10,000 lux and 15:1 at 20,000 lux help preserve darker structures. -
Specular Reflection3
Mirror-like reflections from the surface should be kept below ~0.8% using optical bonding and matched AR coatings to reduce glare from surgical lights. -
Diffuse Reflection
Scattered light should be minimized (often <0.5%) to avoid a hazy image and preserve detail in darker regions. -
Veiling Glare Resistance
Optical bonding eliminates the air gap where internal scattering occurs, helping maintain dark-area detail under bright illumination.
| Optical Performance Metric | Standard Display | Basic Optical Bonding | Premium Medical Optical Bonding | Clinical Impact |
|---|---|---|---|---|
| Ambient Contrast Ratio (10,000 lux) | 10:1 | 20:1 | >30:1 | Structure visibility in bright environments |
| Specular Reflection | 4–5% | 1–2% | <0.8% | Reduction of mirror-like reflections from lights |
| Diffuse Reflection | 2–3% | 0.7–1% | <0.5% | Preservation of detail in darker image regions |
| Color Accuracy (ΔE) | >4 | 2–3 | <2 | Accurate tissue differentiation |
| Uniformity | 75–80% | 85–90% | >92% | Consistent interpretation across screen |
How should you evaluate the bonding process and quality control behind the medical monitor?
Some OEM teams only discover process weaknesses months after SOP, when bubble rates rise or cleaning damage begins to appear in the field.
For optical-bonded OEM monitors, the real risk is not the first 10 nice-looking samples but the next 10,000 units—so I audit bonding lines for process stability, traceability, and medical-grade validation.

The long-term quality of an optical-bonded medical display4 depends far more on disciplined manufacturing than on adhesive brand names. When I visit a bonding supplier, I look for cleanroom control, material handling and traceability, automated dispensing, and validated curing profiles rather than just polished demo parts. I also check how thoroughly the process has been tested for temperature and humidity cycling, transport vibration, and resistance to hospital disinfectants. Suppliers who can show mature validation reports and stable SPC trends are far more likely to deliver consistent quality throughout the device lifecycle.
Process Stability Indicators
Key manufacturing elements that predict consistent bonding quality:
-
Environmental Control Systems
ISO Class 7 or better cleanroom conditions with continuous monitoring of temperature, humidity, and particulates. -
Material Control and Traceability
Tight control of adhesive shelf life, mixing ratios, and batch records to support root cause analysis. -
Automated Dispensing Systems
Computer-controlled adhesive dispensing with vision verification instead of manual application. -
Lamination Technology
Vacuum or controlled-pressure lamination that minimizes bubbles and ensures uniform contact. -
Curing Protocol Validation
Documented curing profiles with validated temperature and UV exposure for repeatable bond strength.
Quality Assurance Framework
Quality systems tailored to optical bonding should include:
- Automated Optical Inspection for bubbles, particles, and edge defects
- Environmental Testing such as temperature cycling, humidity, and vibration
- Chemical Compatibility Testing against common hospital disinfectants
- Statistical Process Control on critical parameters to detect drift early
What mechanical, thermal, and integration factors for bonded displays can OEMs not ignore?
Many product teams assume a bonded display can simply replace a non-bonded one, until late-stage tests reveal mounting, sealing, or thermal issues that force redesigns.
Once you bond glass to the LCD, the display becomes a new mechanical and thermal component, so I always re-validate fit, strength, and heat behavior instead of assuming drop-in compatibility.
Optical bonding changes thickness, weight, stiffness, and heat flow in ways that ripple through the whole device. The added glass and adhesive increase stack height and mass, affecting bezels, gaskets, hinges, mounts, and cable routing. Stress distributions shift under vibration and impact, and the altered thermal path5 can raise panel temperatures unless cooling is updated. For mobile systems, the new mass and center of gravity affect stability and shock performance. Treating the optical-bonded OEM medical monitor as a new part and validating it with updated drop, vibration, and thermal tests is the safest way to avoid late redesigns and field failures.
Mechanical Integration Considerations
Optical bonding transforms the physical properties of the display:
-
Dimensional Changes
Added thickness affects bezels, seals, and clearances; early 3D data is essential. -
Weight Implications
Higher mass influences mounts, hinges, and cart or arm stability. -
Stress Distribution
Composite behavior can concentrate loads at mounting points and edges. -
Expansion and Contraction
Different CTEs of glass, adhesive, and LCD require compliant mounting. -
Connector and Cable Access
Thicker assemblies change connector reach, bend radii, and service access.
Thermal Management Implications
Bonding also alters thermal behavior:
- Modified Heat Dissipation Paths that reduce front-side heat flow
- Edge Temperature Gradients affecting uniformity and stress
- Internal Condensation Reduction when sealing is done correctly
- Lifetime Aging Effects if higher panel temperatures are not managed
How should device manufacturers evaluate vendors and long-term partnership risk for OEM medical monitors?
Because medical device lifecycles run for many years, the wrong bonding partner can turn every panel change into a regulatory and supply-chain crisis.
In my OEM work, the best optical-bonding partners are rarely the cheapest; they are the ones who plan for panel changes, regulatory impact, and lifecycle support over 7–10 years.
Selecting a supplier for optical-bonded OEM medical monitors is a strategic decision that must align with your device roadmap and regulatory obligations. When I assess partners, I look at how vertically integrated they are, how much of their business is truly medical, and how many engineers support bonding and integration. I also examine lifecycle and change-control processes, plus the level of documentation and validation support they can provide when something in the display stack evolves. For device manufacturers, a vendor who can minimize revalidation impact and handle transitions gracefully often creates more value than one offering the lowest unit price.
Strategic Partnership Indicators
Characteristics that signal long-term partnership viability:
- Vertical Integration Depth across panel selection, bonding, assembly, and test
- Medical Industry Focus with a significant share of business in healthcare
- Engineering Resource Allocation for optical bonding and medical integration
- Component Lifecycle Management with structured panel EOL processes
- Quality System Maturity beyond basic ISO certification
- Regulatory & Validation Support to manage display changes with minimal re-testing
Risk Mitigation Strategies
Formal practices that reduce lifecycle risk:
- Detailed Supply Agreements specifying change notice periods and support durations
- Second-Source Development for key configurations
- Regular Business Reviews covering technology roadmaps and lifecycle planning
- Design for Transition so future display changes require minimal mechanical and regulatory rework
Which Reshin optical-bonded OEM monitors can serve different device scenarios?
Even with clear targets, device teams often struggle to translate clinical workflows into concrete choices for size, resolution, interfaces, and bonding level.
In my surgical and interventional projects, I start monitor selection from real device scenarios—endoscopy carts, towers, hybrid ORs, and teaching setups—and then match them to Reshin’s optical-bonded OEM platforms.
Different clinical workflows place different demands on the display. Compact endoscopy systems need high-PPI 27″ 4K panels with AR glass and flexible inputs. Standard surgical towers benefit from 31.5–32″ 4K monitors with wide viewing angles and SDI support. Ceiling-mounted hybrid ORs require bright 32″ 4K screens with strong reflection control, while team-viewing and teaching setups call for 42.5–55″ displays that stay readable from across the room. Starting from these usage patterns and then tailoring mechanics, interfaces, and branding lets OEMs build differentiated systems on top of proven Reshin platforms.
| Clinical Application | Recommended Models | Key Characteristics | Integration Considerations |
|---|---|---|---|
| Compact Endoscopy Systems | MS275PA (27" 4K) | High pixel density (163 PPI), AR-treated glass, Multiple digital inputs (HDMI/DP) | Limited footprint, Integration with camera systems, Portrait/landscape flexibility |
| Standard Surgical Towers | MS321PB (31.5" 4K) | Wide viewing angles (178°), Robust optical bonding, Rich I/O including SDI | Structured cable management, Standard VESA mounting |
| Ceiling-mounted Hybrid OR | MS322PB (32" 4K) | Long-distance signal support, Multiple simultaneous inputs, Enhanced brightness | Remote mounting considerations, Signal integrity over distance |
| Team Viewing / Teaching | MS430PC (42.5" 4K) | Large optical-bonded surface, Anti-reflection coating, Multi-input capability | Mounting weight support, Viewing distance optimization |
| Team Viewing / Teaching | MS550P (55" 4K) | Large optical-bonded surface, Anti-reflection coating, Multi-input capability | Mounting weight support, Viewing distance optimization |
When integrating these displays, I work with OEM teams to tune color behavior for endoscopy, ensure brightness and contrast targets suit hybrid OR lighting, and adapt mechanics and interfaces to fit proprietary enclosures. This combination of standard platforms plus focused customization lets device manufacturers control risk while still delivering systems that feel unique to their brand and clinical users.
FAQ: Optical-bonded OEM medical monitors
Do all medical devices need optical-bonded monitors?
No. Optical bonding delivers the most value in bright, high-ambient environments, mobile systems, and applications with frequent cleaning or fluid exposure. In low-light, fixed setups, non-bonded options may be sufficient.
How much more expensive is an optical-bonded medical display?
Optical-bonded OEM medical monitors usually carry a noticeable premium. However, when reduced field failures, higher clinical usability, and longer service life are considered, lifecycle cost can be lower than for non-bonded solutions.
Can I switch optical bonding suppliers without revalidation?
Usually not completely. Changing bonding suppliers or processes typically triggers at least partial revalidation. Vendors with strong regulatory and validation support can help minimize test scope and documentation work.
Conclusion
Selecting an optical-bonded OEM medical monitor is a system-level decision that connects optical performance, mechanical and thermal behavior, manufacturing quality, and lifecycle strategy. Device manufacturers that define realistic clinical metrics, audit bonding processes, and treat bonded displays as new subsystems—rather than drop-in parts—are far less likely to encounter costly redesigns, field failures, and supply disruptions.
Reshin is a specialized China-based manufacturer of medical-grade displays and optical-bonded OEM monitors, working with device makers on engineering customization, lifecycle planning, and validation-friendly transitions. If your team is planning a new device platform or redesign involving optical-bonded medical monitors, our engineering group can help translate your clinical and regulatory requirements into a concrete display configuration and integration plan.
📧 info@reshinmonitors.com
🌐 https://reshinmonitors.com/
-
Understanding optical bonding can enhance your knowledge of its impact on image quality and device reliability. ↩
-
Learn about the importance of infection control in medical devices and how cleanability can enhance patient safety. ↩
-
Exploring Specular Reflection helps in grasping how glare control can improve surgical precision and visual clarity. ↩
-
Exploring this link will provide insights into the advantages and applications of optical-bonded medical displays in improving healthcare outcomes. ↩
-
Exploring the thermal path can help in improving cooling solutions and preventing overheating in devices. ↩



